Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus
- PMID: 11090176
- PMCID: PMC112459
- DOI: 10.1128/jvi.74.24.11764-11772.2000
Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus
Abstract
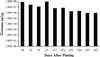
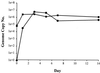
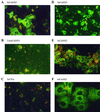
GB virus-B (GBV-B) causes an acute hepatitis in tamarins characterized by increased alanine transaminase levels that quickly return to normal as the virus is cleared. Phylogenetically, GBV-B is the closest relative to hepatitis C virus (HCV), and thus GBV-B infection of tamarins represents a powerful surrogate model system for the study of HCV. In this study, the course of infection of GBV-B in tamarins was followed using a real-time 5' exonuclease (TaqMan) reverse transcription-PCR assay to determine the level of GBV-B in the serum. Peak viremia levels exceeded 10(9) genome equivalents/ml, followed by viral clearance within 14 to 16 weeks. Rechallenge of animals that had cleared infection resulted in viremia that was limited to 1 week, suggestive of a strong protective immune response. A robust tissue culture system for GBV-B was developed using primary cultures of tamarin hepatocytes. Hepatocytes obtained from a GBV-B-infected animal maintained high levels of cell-associated viral RNA and virion secretion for 42 days of culture. In vitro infection of normal hepatocytes resulted in rapid amplification of cell-associated viral RNA and secretion of up to 10(7) genome equivalents/ml of culture supernatant. In addition, infection could be monitored by immunofluorescence staining for GBV-B nonstructural NS3 protein. This model system overcomes many of the current obstacles to HCV research, including low levels of viral replication, lack of a small primate animal model, and lack of a reproducible tissue culture system.
Figures



References
-
- Alter H, Nakatsuji Y, Melpolder J, Wages J, Jr, Wesley R, Shih J W K, Kim J P. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–754. - PubMed
-
- Beard M R, Abell G, Honda M, Carroll A, Gartland M, Clarke B, Suzuki K, Lanford R, Sangar D V, Lemon S M. An infectious molecular clone of a Japanese genotype 1b hepatitis C virus. Hepatology. 1999;30:316–324. - PubMed
-
- Bronowicki J P, Vetter D, Uhl G, Hudziak H, Uhrlacher A, Vetter J M, Doffoel M. Lymphocyte reactivity to hepatitis C virus (HCV) antigens shows evidence for exposure to HCV in HCV-seronegative spouses of HCV-infected patients. J Infect Dis. 1997;176:518–522. - PubMed
-
- Bukh J, Apgar C L. Five new or recently discovered (GBV-A) virus species are indigenous to New World monkeys and may constitute a separate genus of the Flaviviridae. Virology. 1997;229:429–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

