Lytic but not latent replication of epstein-barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins
- PMID: 11090180
- PMCID: PMC112463
- DOI: 10.1128/jvi.74.24.11800-11810.2000
Lytic but not latent replication of epstein-barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins
Abstract
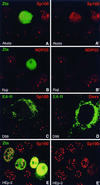
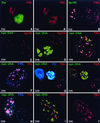
Nuclear domains called ND10 (nuclear domain 10) are discrete nuclear protein aggregations characterized by a set of interferon-upregulated proteins including Sp100 and PML, where papova-, adeno-, and herpesviruses begin their transcription and DNA replication. Both the alpha- and betaherpesvirus subfamilies disrupt ND10 upon infection by dispersing and/or destroying ND10-associated proteins. We studied the effect of the gammaherpesvirus Epstein-Barr virus (EBV) on ND10 and its spatial distribution in the nucleus of cells during latency and lytic reactivation. In latently infected Burkitt's lymphoma, lymphoblastoid, and D98/HR1 cells, ND10 were intact, as judged by immunofluorescence localization of PML, Sp100, NDP55, and Daxx. Fluorescent in situ hybridization revealed no association between viral episomes and ND10 during latency, implying that the maintenance replication of EBV, which depends on host cell proliferation, occurs independent of ND10. As in mitosis, the EBV genomes were attached to interphase chromosomes, suggesting that they are unable to move freely within the interchromosomal space and thus unable to associate with the interchromosomally located ND10 or other nuclear domains. Upon lytic activation, ND10 became dispersed in cells expressing lytic proteins. Redistribution of ND10 proteins occurred sequentially at different stages of the lytic cycle, with Sp100, Daxx, and NDP55 dispersed before and PML dispersed after the onset of lytic replication. ND10 remnants were retained until the early stages of lytic replication, and replicating EBV genomes were frequently found beside this nuclear domain; the number of replication domains was usually lower than the average latent virus frequency. Thus, latency does not require or induce interaction of EBV with ND10 for transcription and replication, whereas lytic replication triggers dispersion of ND10 proteins and occurs in close association with PML aggregates. The required movement of chromosome-attached latent EBV episomes to ND10 after reactivation from latency might include physical release of the chromosome-bound episomes. Only episomes contacting ND10 after such a release might be able to begin lytic replication.
Figures


References
-
- Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, et al. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

