Sialic acid species as a determinant of the host range of influenza A viruses
- PMID: 11090182
- PMCID: PMC112465
- DOI: 10.1128/jvi.74.24.11825-11831.2000
Sialic acid species as a determinant of the host range of influenza A viruses
Abstract
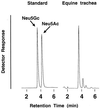
The distribution of sialic acid (SA) species varies among animal species, but the biological role of this variation is largely unknown. Influenza viruses differ in their ability to recognize SA-galactose (Gal) linkages, depending on the animal hosts from which they are isolated. For example, human viruses preferentially recognize SA linked to Gal by the alpha2,6(SAalpha2,6Gal) linkage, while equine viruses favor SAalpha2,3Gal. However, whether a difference in relative abundance of specific SA species (N-acetylneuraminic acid [NeuAc] and N-glycolylneuraminic acid [NeuGc]) among different animals affects the replicative potential of influenza viruses is uncertain. We therefore examined the requirement for the hemagglutinin (HA) for support of viral replication in horses, using viruses whose HAs differ in receptor specificity. A virus with an HA recognizing NeuAcalpha2,6Gal but not NeuAcalpha2,3Gal or NeuGcalpha2,3Gal failed to replicate in horses, while one with an HA recognizing the NeuGcalpha2,3Gal moiety replicated in horses. Furthermore, biochemical and immunohistochemical analyses and a lectin-binding assay demonstrated the abundance of the NeuGcalpha2,3Gal moiety in epithelial cells of horse trachea, indicating that recognition of this moiety is critical for viral replication in horses. Thus, these results provide evidence of a biological effect of different SA species in different animals.
Figures
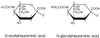


References
-
- Baum L G, Paulson J C. The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology. 1991;180:10–15. - PubMed
-
- Bean W J, Sriram G, Webster R G. Electrophoretic analysis of iodine-labeled influenza RNA segments. Anal Biochem. 1980;102:228–232. - PubMed
-
- Beare A S, Webster R G. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. - PubMed
-
- Chambers T M, Shortridge K F, Li P H, Powell D G, Watkins K L. Rapid diagnosis of equine influenza by the Directigen FLU-A enzyme immunoassay. Vet Rec. 1994;135:275–279. - PubMed
-
- Claas E C J, Osterhaus A D M E, Van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

