Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family
- PMID: 11090195
- PMCID: PMC112478
- DOI: 10.1128/jvi.74.24.11950-11954.2000
Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family
Abstract
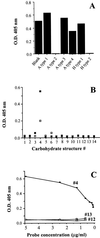
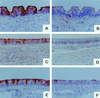
The ability of rabbit hemorrhagic disease virus to agglutinate human erythrocytes and to attach to rabbit epithelial cells of the upper respiratory and digestive tracts was shown to depend on the presence of ABH blood group antigens. Indeed, agglutination was inhibited by saliva from secretor individuals but not from nonsecretors, the latter being devoid of H antigen. In addition, erythrocytes of the rare Bombay phenotype, which completely lack ABH antigens, were not agglutinated. Native viral particles from extracts of infected rabbit liver as well as virus-like particles from the recombinant virus capsid protein specifically bound to synthetic A and H type 2 blood group oligosaccharides. Both types of particles could attach to adult rabbit epithelial cells of the upper respiratory and digestive tracts. This binding paralleled that of anti-H type 2 blood group reagents and was inhibited by the H type 2-specific lectin UEA-I and polyacrylamide-conjugated H type 2 trisaccharide. Young rabbit tissues were almost devoid of A and H type 2 antigens, and only very weak binding of virus particles could be obtained on these tissues.
Figures
References
-
- Breimer M E, Hansson G C, Karlsson K A, Leffler H, Pimlot W, Samuelsson B E. Selected ion monitoring of glycosphingolipid mixtures. Identification of several blood group type glycolipids in the small intestine of an individual rabbit. Biomed Mass Spectrom. 1979;6:231–241. - PubMed
-
- Capucci L, Scicluna M T, Lavazza A. Diagnosis of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech Off Int Epizoot. 1991;10:347–370. - PubMed
-
- Clarke I N, Lambden P R. The molecular biology of caliciviruses. J Gen Virol. 1997;78:291–301. - PubMed
-
- Fukuda M N, Levery S B. Glycolipids of fetal, newborn and adult erythrocytes: glycolipid pattern and structural study of H3-glycolipid from newborn erythrocytes. Biochemistry. 1983;22:5034–5040. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

