Cell wall alterations in the arabidopsis emb30 mutant
- PMID: 11090208
- PMCID: PMC150157
- DOI: 10.1105/tpc.12.11.2047
Cell wall alterations in the arabidopsis emb30 mutant
Abstract
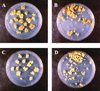
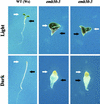
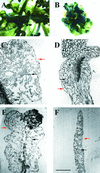
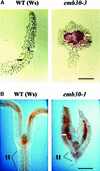
The Arabidopsis EMB30 gene is essential for controlling the polarity of cell growth and for normal cell adhesion during seedling development. In this article, we show that emb30 mutations also affect the growth of undifferentiated plant cells and adult tissues. EMB30 possesses a Sec7 domain and, based on similarities to other proteins, presumably functions in the secretory pathway. The plant cell wall depends on the secretory pathway to deliver its complex polysaccharides. We show that emb30 mutants have a cell wall defect that sometimes allows material to be deposited into the interstitial space between cells instead of being restricted to cell corners. In addition, pectin, a complex polysaccharide important for cell adhesion, appears to be abnormally localized in emb30 plants. In contrast, localization of epitopes associated with xyloglucan or arabinogalactan was similar in wild-type and emb30 tissues, and the localization of a marker molecule to vacuoles appeared normal. Therefore, emb30 mutations do not cause a general defect in the secretory pathway. Together, these results suggest that emb30 mutations result in an abnormal cell wall, which in turn may account for the defects in cell adhesion and polar cell growth control observed in the mutants.
Figures

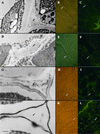
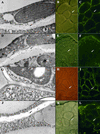
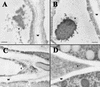
References
-
- Baus, A.D., Franzmann, L., and Meinke, D.W. (1986). Growth in vitro of arrested embryos from lethal mutants of Arabidopsis thaliana. Theor. Appl. Genet. 72, 577–586. - PubMed
-
- Belanger, K.D., and Quatrano, R.S. (2000). Polarity: The role of localized secretion. Curr. Opin. Plant Biol. 3, 67–72. - PubMed
-
- Bouget, F., Berger, F., and Brownlee, C. (1998). Position dependent control of cell fate in the Fucus embryo: Role of intercellular communication. Development 125, 1999–2008. - PubMed
-
- Busch, M., Mayer, U., and Jürgens, G. (1996). Molecular analysis of the Arabidopsis pattern formation gene GNOM: Gene structure and intragenic complementation. Mol. Gen. Genet. 250, 681–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

