Tobacco VDL gene encodes a plastid DEAD box RNA helicase and is involved in chloroplast differentiation and plant morphogenesis
- PMID: 11090214
- PMCID: PMC150163
- DOI: 10.1105/tpc.12.11.2129
Tobacco VDL gene encodes a plastid DEAD box RNA helicase and is involved in chloroplast differentiation and plant morphogenesis
Abstract
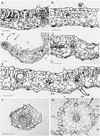
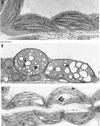
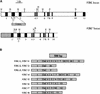
The recessive nuclear vdl (for variegated and distorted leaf) mutant of tobacco was obtained by T-DNA insertion and characterized by variegated leaves and abnormal roots and flowers. Affected leaf tissues were white and distorted, lacked palisadic cells, and contained undifferentiated plastids. The variegation was due to phenotypic, rather than genetic, instability. Genomic and cDNA clones were obtained for both the mutant and wild-type VDL alleles. Three transcripts, resulting from alternate intron splicing or polyadenylation, were found for the wild type. The transcripts potentially encode a set of proteins (53, 19, and 15 kD) sharing the same N-terminal region that contains a chloroplast transit peptide capable of importing the green fluorescent protein into chloroplasts. The predicted 53-kD product belongs to the DEAD box RNA helicase family. In the homozygous vdl mutant, T-DNA insertion resulted in accumulation of the shortest transcript and the absence of the RNA helicase-encoding transcript. Genetic transformation of the homozygous mutant by the 53-kD product-encoding cDNA fully restored the wild-type phenotype. These data suggest that a plastid RNA helicase controls early plastid differentiation and plant morphogenesis.
Figures





References
-
- Amaratunga, M., and Lohman, T.M. (1993). Escherichia coli rep helicase unwinds DNA by an active mechanism. Biochemistry 32, 6815–6820. - PubMed
-
- Bird, L.E., Subramanya, H.S., and Wigley, D.B. (1998). Helicases: A unifying structural theme? Curr. Opin. Struct. Biol. 8, 14–18. - PubMed
-
- Cabrera y Poch, H.L., Peto, C.A., and Chory, J. (1993). A mutation in the Arabidopsis DET3 gene uncouples photoregulated leaf development from gene expression and chloroplast biogenesis. Plant J. 4, 671–682.
-
- Carol, P., Stevenson, D., Bisanz, C., Breitenbach, J., Sandmann, G., Mache, R., Coupland, G., and Kuntz, M. (1999). Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11, 57–68. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

