Shoot organization genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice
- PMID: 11090216
- PMCID: PMC150165
- DOI: 10.1105/tpc.12.11.2161
Shoot organization genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice
Abstract
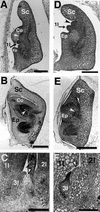
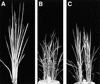
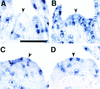
The mechanism regulating the pattern of leaf initiation was analyzed by using shoot organization (sho) mutants derived from three loci (SHO1, SHO2, and SHO3). In the early vegetative phase, sho mutants show an increased rate of leaf production with random phyllotaxy. The resulting leaves are malformed, threadlike, or short and narrow. Their shoot apical meristems are relatively low and wide, that is, flat shaped, although their shape and size are highly variable among plants of the same genotype. Statistical analysis reveals that the shape of the shoot meristem rather than its size is closely correlated with the variations of plastochron and phyllotaxy. Rapid and random leaf production in sho mutants is correlated with the frequent and disorganized cell divisions in the shoot meristem and with a reduction of expression domain of a rice homeobox gene, OSH1. These changes in the organization and behavior of the shoot apical meristems suggest that sho mutants have fewer indeterminate cells and more determinate cells than wild type, with many cells acting as leaf founder cells. Thus, the SHO genes have an important role in maintaining the proper organization of the shoot apical meristem, which is essential for the normal initiation pattern of leaf primordia.
Figures





Comment in
-
The use of entropy to analyze phyllotactic mutants: a theoretical analysis.Plant Cell. 2004 Apr;16(4):804-6. doi: 10.1105/tpc.160440. Plant Cell. 2004. PMID: 15064366 Free PMC article. No abstract available.
References
-
- Barton, M.K., and Poethig, R.S. (1993). Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 119, 823–831.
-
- Bowman, J.L. (1994). Arabidopsis, an Atlas of Morphology and Development. (New York: Springer-Verlag).
-
- Callos, J.D., and Medford, J.I. (1994). Organ position and pattern formation in the shoot apex. Plant J. 6, 1–7.
-
- Callos, J.D., Dirado, M., Xu, B., Behringer, F.J., Link, B.M., and Medford, J.I. (1994). The forever young gene encodes an oxidoreductase required for proper development of the Arabidopsis vegetative shoot apex. Plant J. 6, 835–847. - PubMed
-
- Chaudhury, A.M., Letham, S., Craig, S., and Dennis, E.S. (1993). amp1—A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 4, 907–916.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

