A rab1 GTPase is required for transport between the endoplasmic reticulum and golgi apparatus and for normal golgi movement in plants
- PMID: 11090219
- PMCID: PMC150168
- DOI: 10.1105/tpc.12.11.2201
A rab1 GTPase is required for transport between the endoplasmic reticulum and golgi apparatus and for normal golgi movement in plants
Abstract
We describe a green fluorescent protein (GFP)-based assay for investigating membrane traffic on the secretory pathway in plants. Expression of AtRab1b(N121I), predicted to be a dominant inhibitory mutant of the Arabidopsis Rab GTPase AtRab1b, resulted in accumulation of a secreted GFP marker in an intracellular reticulate compartment reminiscent of the endoplasmic reticulum. This accumulation was alleviated by coexpressing wild-type AtRab1b but not AtRab8c. When a Golgi-targeted and N-glycosylated variant of GFP was coexpressed with AtRab1b(N121I), the variant also accumulated in a reticulate network and an endoglycosidase H-sensitive population appeared. Unexpectedly, expression of AtRab1b(N121I), but not of the wild-type AtRab1b, resulted in a reduction or cessation of vectorial Golgi movement, an effect that was reversed by coexpression of the wild type. We conclude that AtRab1b function is required for transport from the endoplasmic reticulum to the Golgi apparatus and suggest that this process may be coupled to the control of Golgi movement.
Figures



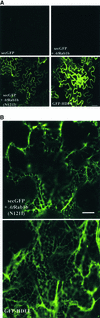
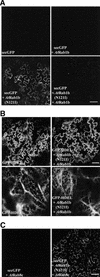
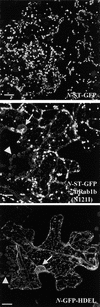
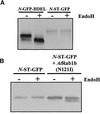

Comment in
-
Green light for traffic in the early secretory pathway.Plant Cell. 2000 Nov;12(11):2009-11. doi: 10.1105/tpc.12.11.2009. Plant Cell. 2000. PMID: 11090204 Free PMC article. No abstract available.
References
-
- Ausubel, F., Brent, R., Kingston, R.E., Moore, J.G., Seidman, J.G., Smith, J.A., and Struhl, J.G. (1999). Current Protocols in Molecular Biology. (New York: John Wiley).
-
- Barbacid, M. (1987). ras genes. Annu. Rev. Biochem. 56, 779–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

