In situ localization and in vitro induction of plant COPI-coated vesicles
- PMID: 11090220
- PMCID: PMC150169
- DOI: 10.1105/tpc.12.11.2219
In situ localization and in vitro induction of plant COPI-coated vesicles
Abstract
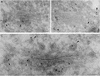
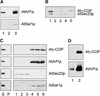
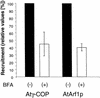
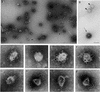
Coat protein (COP)-coated vesicles have been shown to mediate protein transport through early steps of the secretory pathway in yeast and mammalian cells. Here, we attempt to elucidate their role in vesicular trafficking of plant cells, using a combined biochemical and ultrastructural approach. Immunogold labeling of cryosections revealed that COPI proteins are localized to microvesicles surrounding or budding from the Golgi apparatus. COPI-coated buds primarily reside on the cis-face of the Golgi stack. In addition, COPI and Arf1p show predominant labeling of the cis-Golgi stack, gradually diminishing toward the trans-Golgi stack. In vitro COPI-coated vesicle induction experiments demonstrated that Arf1p as well as coatomer could be recruited from cauliflower cytosol onto mixed endoplasmic reticulum (ER)/Golgi membranes. Binding of Arf1p and coatomer is inhibited by brefeldin A, underlining the specificity of the recruitment mechanism. In vitro vesicle budding was confirmed by identification of COPI-coated vesicles through immunogold negative staining in a fraction purified from isopycnic sucrose gradient centrifugation. Similar in vitro induction experiments with tobacco ER/Golgi membranes prepared from transgenic plants overproducing barley alpha-amylase-HDEL yielded a COPI-coated vesicle fraction that contained alpha-amylase as well as calreticulin.
Figures






Comment in
-
Green light for traffic in the early secretory pathway.Plant Cell. 2000 Nov;12(11):2009-11. doi: 10.1105/tpc.12.11.2009. Plant Cell. 2000. PMID: 11090204 Free PMC article. No abstract available.
References
-
- Andreeva, A.V., Kutuzov, M.A., Evans, D.E., and Hawes, C.R. (1998). The structure and function of the Golgi apparatus: A hundred years of questions. J. Exp. Bot. 49, 1281–1291.
-
- Bannykh, S.I., and Balch, W.E. (1998). Selective transport of cargo between the endoplasmic reticulum and Golgi compartments. Histochem. Cell Biol. 109, 463–475. - PubMed
-
- Barlowe, C., and Schekman, R. (1993). SEC12 encodes a guanine nucleotide exchange factor essential for transport vesicle formation from the ER. Nature 365, 347–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

