Structural basis of SNT PTB domain interactions with distinct neurotrophic receptors
- PMID: 11090629
- PMCID: PMC5155437
- DOI: 10.1016/s1097-2765(05)00087-0
Structural basis of SNT PTB domain interactions with distinct neurotrophic receptors
Abstract
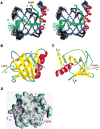
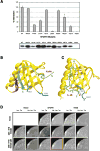
SNT adaptor proteins transduce activation of fibroblast growth factor receptors (FGFRs) and neurotrophin receptors (TRKs) to common signaling targets. The SNT-1 phosphotyrosine binding (PTB) domain recognizes activated TRKs at a canonical NPXpY motif and, atypically, binds to nonphosphorylated FGFRs in a region lacking tyrosine or asparagine. Here, using NMR and mutational analyses, we show that the PTB domain utilizes distinct sets of amino acid residues to interact with FGFRs or TRKs in a mutually exclusive manner. The FGFR1 peptide wraps around the beta sandwich structure of the PTB domain, and its binding is possibly regulated by conformational change of a unique C-terminal beta strand in the protein. Our results suggest mechanisms by which SNTs serve as molecular switches to mediate the essential interplay between FGFR and TRK signaling during neuronal differentiation.
Figures


References
-
- Birren SJ, Anderson DJ. A v-myc immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron. 1990;4:189–201. - PubMed
-
- Blaikie P, Immanuel D, Wu J, Li N, Yajnik V, Margolis B. A region in Shc distinct from the SH2 domain can bind a tyrosine phosphorylated growth factor receptor. J Biol Chem. 1994;269:32031–32034. - PubMed
-
- Brünger AT. X-PLOR Version 3.1:ASystem for X-Ray Crystallography and NMR, Version 3.1 Edition. New Haven, CT: Yale University Press; 1993.
-
- Carson M. Ribbons 2.0. J Appl Crystallogr. 1991;24:958–961.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

