Pleiotropic effect of protein P6 on the viral cycle of bacteriophage phi29
- PMID: 11092852
- PMCID: PMC94817
- DOI: 10.1128/JB.182.24.6927-6932.2000
Pleiotropic effect of protein P6 on the viral cycle of bacteriophage phi29
Abstract
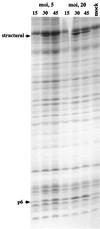
The product of bacteriophage phi29 early gene 6, protein p6, is a double-stranded-DNA binding protein and one of the more abundant proteins during viral infection. We have studied the role of protein p6 in vivo through the infection of suppressor and nonsuppressor Bacillus subtilis strains with a phage carrying a nonsense mutation in gene 6, sus6(626). In the absence of functional protein p6, the two major processes of the viral cycle, transcription and DNA replication, were affected. Viral DNA synthesis was practically abolished, and early transcription was remarkably delayed and, in addition, underregulated at late times of the infection. The amount of protein p6 synthesized after infection with mutant phage sus6(626) under suppressor conditions was sixfold lower than that produced after wild-type infection. Nonetheless, phage production was as high as that obtained after wild-type infection. These results indicate that p6 is synthesized in amounts higher than those needed for most of its functions. However, the concentration of protein p6 appeared to be important for repression of the early promoter C2.
Figures


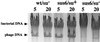



References
-
- Abril A, Salas M, Andreu J M, Hermoso J M, Rivas G. Phage φ29 protein p6 is in a monomer-dimer equilibrium that shifts to higher association states at the millimolar concentration found in vivo. Biochemistry. 1997;36:11901–11908. - PubMed
-
- Bravo A, Salas M. Initiation of bacteriophage φ29 DNA replication in vivo: assembly of a membrane-associated multiprotein complex. J Mol Biol. 1997;269:102–112. - PubMed
-
- Carrascosa J L, Camacho A, Moreno F, Jiménez F, Mellado R P, Viñuela E, Salas M. Bacillus subtilis bacteriophage φ29. Characterization of gene products and functions. Eur J Biochem. 1976;66:229–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

