c-Myb binds to a sequence in the proximal region of the RAG-2 promoter and is essential for promoter activity in T-lineage cells
- PMID: 11094072
- PMCID: PMC102178
- DOI: 10.1128/MCB.20.24.9203-9211.2000
c-Myb binds to a sequence in the proximal region of the RAG-2 promoter and is essential for promoter activity in T-lineage cells
Abstract
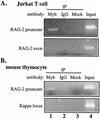
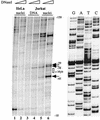
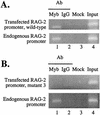
The RAG-2 gene encodes a component of the V(D)J recombinase which is essential for the assembly of antigen receptor genes in B and T lymphocytes. Previously, we reported that the transcription factor BSAP (PAX-5) regulates the murine RAG-2 promoter in B-cell lines. A partially overlapping but distinct region of the proximal RAG-2 promoter was also identified as an important element for promoter activity in T cells; however, the responsible factor was unknown. In this report, we present data demonstrating that c-Myb binds to a Myb consensus site within the proximal promoter and is critical for its activity in T-lineage cells. We show that c-Myb can transactivate a RAG-2 promoter-reporter construct in cotransfection assays and that this transactivation depends on the proximal promoter Myb consensus site. By using a chromatin immunoprecipitation (ChIP) strategy, fractionation of chromatin with anti-c-Myb antibody specifically enriched endogenous RAG-2 promoter DNA sequences. DNase I genomic footprinting revealed that the c-Myb site is occupied in a tissue-specific fashion in vivo. Furthermore, an integrated RAG-2 promoter construct with mutations at the c-Myb site was not enriched in the ChIP assay, while a wild-type integrated promoter construct was enriched. Finally, this lack of binding of c-Myb to a chromosomally integrated mutant RAG-2 promoter construct in vivo was associated with a striking decrease in promoter activity. We conclude that c-Myb regulates the RAG-2 promoter in T cells by binding to this consensus c-Myb binding site.
Figures



Similar articles
-
Lymphoid enhancer-binding factor-1 binds and activates the recombination-activating gene-2 promoter together with c-Myb and Pax-5 in immature B cells.J Immunol. 2002 Oct 1;169(7):3783-92. doi: 10.4049/jimmunol.169.7.3783. J Immunol. 2002. PMID: 12244173
-
Cooperative binding of c-Myb and Pax-5 activates the RAG-2 promoter in immature B cells.Blood. 2002 Jan 15;99(2):576-83. doi: 10.1182/blood.v99.2.576. Blood. 2002. PMID: 11781241
-
Activation of mouse RAG-2 promoter by Myc-associated zinc finger protein.Biochem Biophys Res Commun. 2004 May 14;317(4):1096-102. doi: 10.1016/j.bbrc.2004.03.159. Biochem Biophys Res Commun. 2004. PMID: 15094381
-
Function and control of recombination-activating gene activity.Ann N Y Acad Sci. 1992 May 4;651:277-94. doi: 10.1111/j.1749-6632.1992.tb24626.x. Ann N Y Acad Sci. 1992. PMID: 1599127 Review.
-
Regulation of RAG expression in developing lymphocytes.Curr Opin Immunol. 2000 Apr;12(2):187-90. doi: 10.1016/s0952-7915(99)00070-9. Curr Opin Immunol. 2000. PMID: 10712943 Review.
Cited by
-
High-mobility-group A1 (HMGA1) proteins down-regulate the expression of the recombination activating gene 2 (RAG2).Biochem J. 2005 Jul 1;389(Pt 1):91-7. doi: 10.1042/BJ20041607. Biochem J. 2005. PMID: 15713121 Free PMC article.
-
Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes.J Biol Chem. 2011 Oct 7;286(40):34533-41. doi: 10.1074/jbc.M111.248591. Epub 2011 Aug 16. J Biol Chem. 2011. PMID: 21846717 Free PMC article.
-
Myb proteins regulate expression of histone variant H2A.Z during thymocyte development.Immunology. 2008 Feb;123(2):282-9. doi: 10.1111/j.1365-2567.2007.02697.x. Epub 2007 Oct 11. Immunology. 2008. PMID: 17931383 Free PMC article.
-
Recent insights into the transcriptional control of the Tcra/Tcrd locus by distant enhancers during the development of T-lymphocytes.Transcription. 2015;6(4):65-73. doi: 10.1080/21541264.2015.1078429. Transcription. 2015. PMID: 26230488 Free PMC article. Review.
-
Direct regulation of TCR rearrangement and expression by E proteins during early T cell development.WIREs Mech Dis. 2022 Nov;14(6):e1578. doi: 10.1002/wsbm.1578. Epub 2022 Jul 18. WIREs Mech Dis. 2022. PMID: 35848146 Free PMC article. Review.
References
-
- Agrawal A, Eastman Q, Schatz D G. Transposition mediated by RAG-1 and RAG-2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman I L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Asahina M, Ishiguro N, Wu D, Goryo M, Davis W C, Okada K. The proto-oncogene c-myb is expressed in sporadic bovine lymphoma, but not in enzootic bovine leukosis. J Vet Med Sci. 1996;58:1169–1174. - PubMed
-
- Badiani P, Corbella P, Kioussis D, Marvel J, Weston K. Dominant interfering alleles define a role for c-Myb in T-cell development. Genes Dev. 1994;8:770–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
