Rat protein tyrosine phosphatase eta suppresses the neoplastic phenotype of retrovirally transformed thyroid cells through the stabilization of p27(Kip1)
- PMID: 11094075
- PMCID: PMC102181
- DOI: 10.1128/MCB.20.24.9236-9246.2000
Rat protein tyrosine phosphatase eta suppresses the neoplastic phenotype of retrovirally transformed thyroid cells through the stabilization of p27(Kip1)
Abstract
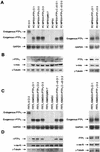
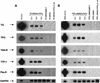
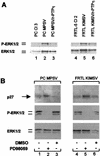
The r-PTPeta gene encodes a rat receptor-type protein tyrosine phosphatase whose expression is negatively regulated by neoplastic cell transformation. Here we first demonstrate a dramatic reduction in DEP-1/HPTPeta (the human homolog of r-PTPeta) expression in a panel of human thyroid carcinomas. Subsequently, we show that the reexpression of the r-PTPeta gene in highly malignant rat thyroid cells transformed by retroviruses carrying the v-mos and v-ras-Ki oncogenes suppresses their malignant phenotype. Cell cycle analysis demonstrated that r-PTPeta caused G(1) growth arrest and increased the cyclin-dependent kinase inhibitor p27(Kip1) protein level by reducing the proteasome-dependent degradation rate. We propose that the r-PTPeta tumor suppressor activity is mediated by p27(Kip1) protein stabilization, because suppression of p27(Kip1) protein synthesis using p27-specific antisense oligonucleotides blocked the growth-inhibitory effect induced by r-PTPeta. Furthermore, we provide evidence that in v-mos- or v-ras-Ki-transformed thyroid cells, the p27(Kip1) protein level was regulated by the mitogen-activated protein (MAP) kinase pathway and that r-PTPeta regulated p27(Kip1) stability by preventing v-mos- or v-ras-Ki-induced MAP kinase activation.
Figures




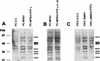

Similar articles
-
An adenovirus carrying the rat protein tyrosine phosphatase eta suppresses the growth of human thyroid carcinoma cell lines in vitro and in vivo.Cancer Res. 2003 Feb 15;63(4):882-6. Cancer Res. 2003. PMID: 12591742
-
The activation of the phosphotyrosine phosphatase eta (r-PTP eta) is responsible for the somatostatin inhibition of PC Cl3 thyroid cell proliferation.Mol Endocrinol. 2001 Oct;15(10):1838-52. doi: 10.1210/mend.15.10.0713. Mol Endocrinol. 2001. PMID: 11579215
-
The rat tyrosine phosphatase eta increases cell adhesion by activating c-Src through dephosphorylation of its inhibitory phosphotyrosine residue.Oncogene. 2005 Apr 28;24(19):3187-95. doi: 10.1038/sj.onc.1208510. Oncogene. 2005. PMID: 15735685
-
Restoration of receptor-type protein tyrosine phosphatase eta function inhibits human pancreatic carcinoma cell growth in vitro and in vivo.Carcinogenesis. 2004 Nov;25(11):2107-14. doi: 10.1093/carcin/bgh224. Epub 2004 Jul 1. Carcinogenesis. 2004. PMID: 15231692
-
BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway.J Biol Chem. 2000 Dec 15;275(50):39223-30. doi: 10.1074/jbc.M007291200. J Biol Chem. 2000. PMID: 11010972
Cited by
-
Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148.J Cell Biol. 2003 May 26;161(4):793-804. doi: 10.1083/jcb.200209019. J Cell Biol. 2003. PMID: 12771128 Free PMC article.
-
The protein tyrosine phosphatase DEP-1/PTPRJ promotes breast cancer cell invasion and metastasis.Oncogene. 2015 Oct 29;34(44):5536-47. doi: 10.1038/onc.2015.9. Epub 2015 Mar 16. Oncogene. 2015. PMID: 25772245
-
Somatic inactivating PTPRJ mutations and dysregulated pathways identified in canine malignant melanoma by integrated comparative genomic analysis.PLoS Genet. 2018 Sep 6;14(9):e1007589. doi: 10.1371/journal.pgen.1007589. eCollection 2018 Sep. PLoS Genet. 2018. PMID: 30188888 Free PMC article.
-
Suppression of the protein tyrosine phosphatase receptor type O gene (PTPRO) by methylation in hepatocellular carcinomas.Oncogene. 2003 Sep 25;22(41):6319-31. doi: 10.1038/sj.onc.1206750. Oncogene. 2003. PMID: 14508512 Free PMC article.
-
CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells.Immunol Rev. 2009 Mar;228(1):288-311. doi: 10.1111/j.1600-065X.2008.00752.x. Immunol Rev. 2009. PMID: 19290935 Free PMC article. Review.
References
-
- Baldassarre G, Belletti B, Spiezia S, Bruni P, Trapasso F, Pentimalli F, Barone M V, Chiappetta G, Vento M T, Boccia A, Fusco A, Viglietto G. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J Clin Investig. 1999;104:865–874. - PMC - PubMed
-
- Brown-Shimer S, Johnson K A, Hill D E, Bruskin A M. Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res. 1992;52:478–482. - PubMed
-
- Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
