Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase pathway
- PMID: 11094087
- PMCID: PMC102193
- DOI: 10.1128/MCB.20.24.9364-9375.2000
Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase pathway
Abstract
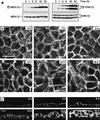
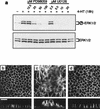
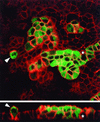
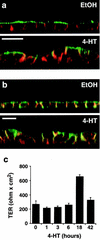
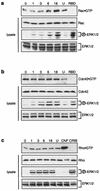
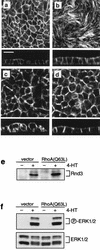
Madin-Darby canine kidney (MDCK) epithelial cells transformed by oncogenic Ras and Raf exhibit cell multilayering and alterations in the actin cytoskeleton. The changes in the actin cytoskeleton comprise a loss of actin stress fibers and enhanced cortical actin. Using MDCK cells expressing a conditionally active form of Raf, we have explored the molecular mechanisms that underlie these observations. Raf activation elicited a robust increase in Rac1 activity consistent with the observed increase in cortical actin. Loss of actin stress fibers is indicative of attenuated Rho function, but no change in Rho-GTP levels was detected following Raf activation. However, the loss of actin stress fibers in Raf-transformed cells was preceded by the induced expression of Rnd3, an endogenous inhibitor of Rho protein function. Expression of Rnd3 alone at levels equivalent to those observed following Raf transformation led to a substantial loss of actin stress fibers. Moreover, cells expressing activated RhoA failed to multilayer in response to Raf. Pharmacological inhibition of MEK activation prevented all of the biological and biochemical changes described above. Consequently, the data are consistent with a role for induced Rnd3 expression downstream of the Raf-MEK-extracellular signal-regulated kinase pathway in epithelial oncogenesis.
Figures
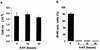

References
-
- Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27894. - PubMed
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. - PubMed
-
- Balkovetz D F, Pollack A L, Mostov K E. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem. 1997;272:3471–3477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
