Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination
- PMID: 11094089
- PMCID: PMC102195
- DOI: 10.1128/MCB.20.24.9391-9398.2000
Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination
Abstract
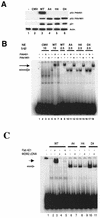
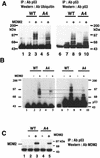
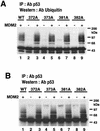
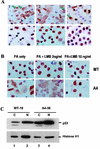
To investigate the effect of mutations in the p53 C-terminal domain on MDM2-mediated degradation, we introduced single and multiple point mutations into a human p53 cDNA at four putative acetylation sites (amino acid residues 372, 373, 381, and 382). Substitution of all four lysine residues by alanines (the A4 mutant) and single lysine-to-alanine substitutions were functional in sequence-specific DNA binding and transactivation; however, the A4 mutant protein was resistant to MDM2-mediated degradation, whereas the single lysine substitutions were not. Although the A4 mutant protein and the single lysine substitutions both bound MDM2 reasonably well, the single lysine substitutions underwent normal MDM2-dependent ubiquitination, whereas the A4 protein was inefficiently ubiquitinated. In addition, the A4 mutant protein was found in the cytoplasm as well as in the nucleus of a subpopulation of cells, unlike wild-type p53, which is mostly nuclear. The partially cytoplasmic distribution of A4 mutant protein was not due to a defect in nuclear import because inhibition of nuclear export by leptomycin B resulted in nuclear accumulation of the protein. Taken together, the data suggest that mutations in the putative acetylation sites of the p53 C-terminal domain interfere with ubiquitination, thereby regulating p53 degradation.
Figures




Similar articles
-
Identification of p53 sequence elements that are required for MDM2-mediated nuclear export.Mol Cell Biol. 2001 Dec;21(24):8533-46. doi: 10.1128/MCB.21.24.8533-8546.2001. Mol Cell Biol. 2001. PMID: 11713288 Free PMC article.
-
Multiple lysine mutations in the C-terminus of p53 make it resistant to degradation mediated by MDM2 but not by human papillomavirus E6 and induce growth inhibition in MDM2-overexpressing cells.Oncogene. 2002 Apr 11;21(16):2605-10. doi: 10.1038/sj.onc.1205343. Oncogene. 2002. PMID: 11971195
-
MDM2 can promote the ubiquitination, nuclear export, and degradation of p53 in the absence of direct binding.J Biol Chem. 2001 Nov 30;276(48):45255-60. doi: 10.1074/jbc.M107477200. Epub 2001 Sep 25. J Biol Chem. 2001. PMID: 11572869
-
The conformationally flexible S9-S10 linker region in the core domain of p53 contains a novel MDM2 binding site whose mutation increases ubiquitination of p53 in vivo.J Biol Chem. 2002 Aug 9;277(32):28446-58. doi: 10.1074/jbc.M202296200. Epub 2002 Mar 29. J Biol Chem. 2002. PMID: 11925449
-
p53 regulation: teamwork between RING domains of Mdm2 and MdmX.Cell Cycle. 2011 Dec 15;10(24):4225-9. doi: 10.4161/cc.10.24.18662. Epub 2011 Dec 15. Cell Cycle. 2011. PMID: 22134240 Review.
Cited by
-
p53 Frameshift Mutations Couple Loss-of-Function with Unique Neomorphic Activities.Mol Cancer Res. 2021 Sep;19(9):1522-1533. doi: 10.1158/1541-7786.MCR-20-0691. Epub 2021 May 27. Mol Cancer Res. 2021. PMID: 34045312 Free PMC article.
-
Impairment of the ubiquitin-proteasome pathway by methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate leads to a potent cytotoxic effect in tumor cells: a novel antiproliferative agent with a potential therapeutic implication.J Biol Chem. 2012 Aug 31;287(36):30625-40. doi: 10.1074/jbc.M111.324228. Epub 2012 Jun 28. J Biol Chem. 2012. PMID: 22745125 Free PMC article.
-
Inhibition of autophagy via p53-mediated disruption of ULK1 in a SCA7 polyglutamine disease model.J Mol Neurosci. 2013 Jul;50(3):586-99. doi: 10.1007/s12031-013-0012-x. Epub 2013 Apr 18. J Mol Neurosci. 2013. PMID: 23592174
-
Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors.Mol Cell Biol. 2003 Apr;23(7):2587-99. doi: 10.1128/MCB.23.7.2587-2599.2003. Mol Cell Biol. 2003. PMID: 12640139 Free PMC article.
-
Identification of p53 sequence elements that are required for MDM2-mediated nuclear export.Mol Cell Biol. 2001 Dec;21(24):8533-46. doi: 10.1128/MCB.21.24.8533-8546.2001. Mol Cell Biol. 2001. PMID: 11713288 Free PMC article.
References
-
- El Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. - PubMed
-
- Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
