Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination
- PMID: 11094089
- PMCID: PMC102195
- DOI: 10.1128/MCB.20.24.9391-9398.2000
Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination
Abstract
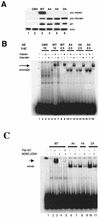
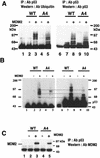
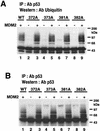
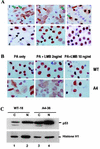
To investigate the effect of mutations in the p53 C-terminal domain on MDM2-mediated degradation, we introduced single and multiple point mutations into a human p53 cDNA at four putative acetylation sites (amino acid residues 372, 373, 381, and 382). Substitution of all four lysine residues by alanines (the A4 mutant) and single lysine-to-alanine substitutions were functional in sequence-specific DNA binding and transactivation; however, the A4 mutant protein was resistant to MDM2-mediated degradation, whereas the single lysine substitutions were not. Although the A4 mutant protein and the single lysine substitutions both bound MDM2 reasonably well, the single lysine substitutions underwent normal MDM2-dependent ubiquitination, whereas the A4 protein was inefficiently ubiquitinated. In addition, the A4 mutant protein was found in the cytoplasm as well as in the nucleus of a subpopulation of cells, unlike wild-type p53, which is mostly nuclear. The partially cytoplasmic distribution of A4 mutant protein was not due to a defect in nuclear import because inhibition of nuclear export by leptomycin B resulted in nuclear accumulation of the protein. Taken together, the data suggest that mutations in the putative acetylation sites of the p53 C-terminal domain interfere with ubiquitination, thereby regulating p53 degradation.
Figures




References
-
- El Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. - PubMed
-
- Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
