Repair of oxidative DNA damage in Drosophila melanogaster: identification and characterization of dOgg1, a second DNA glycosylase activity for 8-hydroxyguanine and formamidopyrimidines
- PMID: 11095666
- PMCID: PMC115177
- DOI: 10.1093/nar/28.23.4583
Repair of oxidative DNA damage in Drosophila melanogaster: identification and characterization of dOgg1, a second DNA glycosylase activity for 8-hydroxyguanine and formamidopyrimidines
Abstract
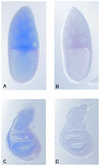
In Drosophila, the S3 ribosomal protein has been shown to act as a DNA glycosylase/AP lyase capable of releasing 8-hydroxyguanine (8-OH-Gua) in damaged DNA. Here we describe a second Drosophila protein (dOgg1) with 8-OH-Gua and abasic (AP) site DNA repair activities. The Drosophila OGG1 gene codes for a protein of 327 amino acids, which shows 33 and 37% identity with the yeast and human Ogg1 proteins, respectively. The DNA glycosylase activity of purified dOgg1 was investigated using gamma-irradiated DNA and gas chromatography/isotope dilution mass spectrometry (GC/IDMS). The dOgg1 protein excises 8-OH-Gua and 2, 6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua) from gamma-irradiated DNA. with k(ca)(t)/K:(M) values of 21.0 x 10(-5) and 11.2 x 10(-5) (min(-1) nM(-1)), respectively. Enzymatic assays using oligodeoxyribonucleotides containing a single lesion show that dOgg1 displays a marked preference for DNA duplexes containing 8-OH-Gua, 8-OH-Ade or an AP site placed opposite a cytosine. The cleavage of the 8-OH-Gua-containing strand results from the excision of the damaged base followed by a ss-elimination reaction at the 3'-side of the resulting AP site. Cleavage of 8-OH-Gua.C duplex involves the formation of a reaction intermediate that is converted into a stable covalent adduct in the presence of sodium borohydre. dOgg1 complements the mutator phenotype of fpg mutY mutants of Escherichia coli. Whole-mount in situ hybridizations on tissues at different stages of Drosophila development reveal that the dOGG1 messenger is expressed uniformly at a low level in cells in which mitotic division occurs. Therefore, Drosophila possesses two DNA glycosylase activities that can excise 8-OH-Gua and formamidopyrimidines from DNA, dOgg1 and the ribosomal protein S3.
Figures





References
-
- Beckman K.B. and Ames,B.N. (1997) Oxidative decay of DNA. J. Biol. Chem., 272, 19633–19636. - PubMed
-
- Boiteux S. and Radicella,J.P. (2000) The human OGG1 gene: structure, functions and its implication in the process of carcinogenesis. Arch. Biochem. Biophys., 377, 1–8. - PubMed
-
- Breimer L.H. (1990) Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol. Carcinog., 3, 188–197. - PubMed
-
- Feig D.I., Reid,T.M. and Loeb,L.A. (1994) Reactive oxygen species in tumorigenesis. Cancer Res., 54, 1890s–1894s. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

