Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity
- PMID: 11095714
- PMCID: PMC17608
- DOI: 10.1073/pnas.240459697
Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity
Abstract
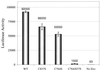
Recent studies suggest that HIV-1 budding occurs selectively from detergent-insoluble membrane domains, referred to as lipid rafts. Palmitoylation is thought to be one of the factors responsible for targeting membrane proteins to lipid rafts. The cytoplasmic domain of the HIV-1 envelope glycoprotein (gp160) contains two palmitoylated cysteine residues. In this work, we studied the solubility of gp160 after detergent extraction. We show that wild-type gp160 is mostly insoluble after ice-cold Triton X-100 extraction, but that it becomes almost completely soluble at 37 degrees C. In contrast, we find that a mutant gp160, in which the two palmitoylated cysteine residues are replaced by serine, is Triton X-100 soluble even under ice-cold extraction. These findings are consistent with the properties of proteins that localize to lipid rafts and strongly suggest that gp160 is associated with lipid rafts. Further, removal of both palmitoylation sites results in the formation of virus with low levels of gp160 incorporation as well as a decrease in viral infectivity by 60-fold. Our results strongly support the suggestion that HIV-1 buds from lipid rafts and point to a role for rafts as a viral assembly hub.
Figures

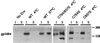

References
-
- Kusumi A, Sako Y. Curr Opin Cell Biol. 1996;8:566–574. - PubMed
-
- Simons K, Ikonen E. Nature (London) 1997;387:569–572. - PubMed
-
- Brown D A, London E. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Schroeder R J, Ahmed S N, Zhu Y, London E, Brown D A. J Biol Chem. 1998;273:1150–1157. - PubMed
-
- Resh M D. Biochim Biophys Acta. 1999;1451:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

