Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin
- PMID: 11095718
- PMCID: PMC17681
- DOI: 10.1073/pnas.240464697
Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin
Abstract
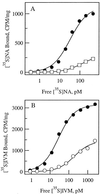
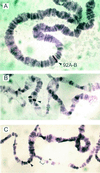
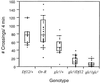
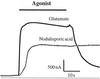
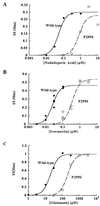
The fruit fly Drosophila melanogaster was used to examine the mode of action of the novel insecticide and acaricide nodulisporic acid. Flies resistant to nodulisporic acid were selected by stepwise increasing the dose of drug in the culture media. The resistant strain, glc(1), is at least 20-fold resistant to nodulisporic acid and 3-fold cross-resistant to the parasiticide ivermectin, and exhibited decreased brood size, decreased locomotion, and bang sensitivity. Binding assays using glc(1) head membranes showed a marked decrease in the affinity for nodulisporic acid and ivermectin. A combination of genetics and sequencing identified a proline to serine mutation (P299S) in the gene coding for the glutamate-gated chloride channel subunit DmGluClalpha. To examine the effect of this mutation on the biophysical properties of DmGluClalpha channels, it was introduced into a recombinant DmGluClalpha, and RNA encoding wild-type and mutant subunits was injected into Xenopus oocytes. Nodulisporic acid directly activated wild-type and mutant DmGluClalpha channels. However, mutant channels were approximately 10-fold less sensitive to activation by nodulisporic acid, as well as ivermectin and the endogenous ligand glutamate, providing direct evidence that nodulisporic acid and ivermectin act on DmGluClalpha channels.
Figures
References
-
- Ostlind D A, Felcetto T, Misura A, Ondeyka J, Smith S, Goetz M, Shoop W, Mickle W. Med Vet Entomol. 1997;11:407–408. - PubMed
-
- Ondeyka J G, Helms G L, Hensens O D, Goetz M A, Zink D L, Tsipouras A, Shoop W L, Slayton L, Dombrowski A W, Polishook J D, et al. J Am Chem Soc. 1997;119:8809–8816.
-
- Smith M M, Warren V A, Thomas B S, Brochu R M, Ertel E A, Rohrer S, Schaeffer J, Schmatz D, Petuch B R, Tang Y S, et al. Biochemistry. 2000;39:5543–5554. - PubMed
-
- Cully D F, Vassilatis D K, Liu K K, Paress P S, Van der Ploeg L H, Schaeffer J M, Arena J P. Nature (London) 1994;371:707–711. - PubMed
-
- Cully D F, Paress P S, Liu K K, Schaeffer J M, Arena J P. J Biol Chem. 1996;271:20187–20191. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

