Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients
- PMID: 11095734
- PMCID: PMC17652
- DOI: 10.1073/pnas.250472097
Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients
Abstract
To evaluate the effects of HIV infection on T cell turnover, we examined levels of DNA synthesis in lymph node and peripheral blood mononuclear cell subsets by using ex vivo labeling with BrdUrd. Compared with healthy controls (n = 67), HIV-infected patients (n = 57) had significant increases in the number and fraction of dividing CD4(+) and CD8(+) T cells. Higher percentages of dividing CD4(+) and CD8(+) T cells were noted in patients with the higher viral burdens. No direct correlation was noted between rates of T cell turnover and CD4(+) T cell counts. Marked reductions in CD4(+) and CD8(+) T cell proliferation were seen in 11/11 patients 1-12 weeks after initiation of highly active antiretroviral therapy (HAART). These reductions persisted for the length of the study (16-72 weeks). Decreases in naive T cell proliferation correlated with increases in the levels of T cell receptor rearrangement excision circles. Division of CD4(+) and CD8(+) T cells increased dramatically in association with rapid increases in HIV-1 viral loads in 9/9 patients 5 weeks after termination of HAART and declined to pre-HAART-termination levels 8 weeks after reinitiation of therapy. These data are consistent with the hypothesis that HIV-1 infection induces a viral burden-related, global activation of the immune system, leading to increases in lymphocyte proliferation.
Figures



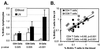
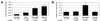
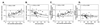

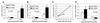
References
-
- Lane H C, Depper J M, Greene W C, Whalen G, Waldmann T A, Fauci A S. N Engl J Med. 1985;313:79–84. - PubMed
-
- Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, et al. Nature (London) 1995;373:117–122. - PubMed
-
- Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Nature (London) 1995;373:123–126. - PubMed
-
- Mosier D E. Nature (London) 1995;375:193–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

