Protein kinase activity of Tel1p and Mec1p, two Saccharomyces cerevisiae proteins related to the human ATM protein kinase
- PMID: 11095737
- PMCID: PMC17647
- DOI: 10.1073/pnas.250475697
Protein kinase activity of Tel1p and Mec1p, two Saccharomyces cerevisiae proteins related to the human ATM protein kinase
Abstract
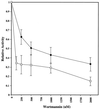
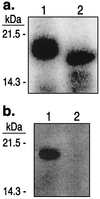
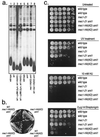
The Saccharomyces cerevisiae proteins Tel1p and Mec1p are involved in telomere length regulation and cellular responses to DNA damage. The closest relative of these proteins is the human Ataxia Telangiectasia Mutated (ATM) protein, a wortmannin-sensitive protein kinase that primarily phosphorylates serines in an SQ motif. We constructed yeast strains containing functional epitope-tagged versions of Tel1p and Mec1p. We showed that immunoprecipitated Tel1p and Mec1p were capable of in vitro phosphorylation of the mammalian protein PHAS-I (Phosphorylated Heat and Acid Stable protein). These activities are sensitive to wortmannin. Tel1p phosphorylates serine in an SQ motif in PHAS-I. Mutations in the kinase domains of Tel1p and Mec1p result in loss of in vitro kinase activity and the in vivo phenotypes associated with the null tel1 and mec1 mutations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

