Pro-coagulant state resulting from high levels of soluble P-selectin in blood
- PMID: 11095738
- PMCID: PMC17662
- DOI: 10.1073/pnas.250475997
Pro-coagulant state resulting from high levels of soluble P-selectin in blood
Abstract
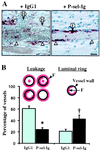
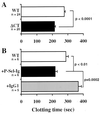
The plasma concentration of soluble adhesion receptors is increased under pathological circumstances, but their function remains enigmatic. Soluble P-selectin (sP-sel) is shed from activated platelets and endothelial cells. Mice genetically engineered to express P-selectin without the cytoplasmic tail (DeltaCT) constitutively show a 3- to 4-fold increase of sP-sel in plasma. We observed that the DeltaCT mice formed fibrin very readily. In an ex vivo perfusion chamber, there was more fibrin deposited at the site of platelet thrombus formation than in wild type (WT), whereas no fibrin deposits were detected using P-selectin-deficient blood during the same interval. Similarly, in vivo, the hemorrhage produced by local Shwartzman reaction was smaller in the DeltaCT mice than in WT. In contrast, we previously showed hemorrhage to be more prominent in P-selectin knock-out mice. Infusion of mouse P-sel-Ig chimera produced the same protective effect in WT mice as seen in the DeltaCT mice, indicating that the effect was due to increased levels of sP-sel. Mice infused with P-sel-Ig showed significantly more fibrin deposited on the luminal face of the injured vessels than control mice. Plasma from DeltaCT mice or mice infused with P-sel-Ig contained higher concentration of pro-coagulant microparticles and clotted one minute faster than WT. This pro-coagulant phenotype of DeltaCT mice could be reversed by a 4-day treatment with PSGL-Ig, a P-selectin inhibitor. We propose that sP-sel should no longer be considered only as a marker of inflammation or platelet activation, but also as a direct inducer of pro-coagulant activity associated with vascular and thrombotic diseases.
Figures


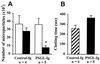
Similar articles
-
Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A.Nat Med. 2003 Aug;9(8):1020-5. doi: 10.1038/nm899. Epub 2003 Jul 13. Nat Med. 2003. PMID: 12858167
-
Endothelial progenitor cells inhibit platelet function in a P-selectin-dependent manner.J Transl Med. 2015 May 7;13:142. doi: 10.1186/s12967-015-0508-y. J Transl Med. 2015. PMID: 25948279 Free PMC article.
-
Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin.J Exp Med. 2003 Jun 2;197(11):1585-98. doi: 10.1084/jem.20021868. J Exp Med. 2003. PMID: 12782720 Free PMC article.
-
Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation.Trends Mol Med. 2004 Apr;10(4):171-8. doi: 10.1016/j.molmed.2004.02.008. Trends Mol Med. 2004. PMID: 15059608 Review.
-
A new role in hemostasis for the adhesion receptor P-selectin.Trends Mol Med. 2004 Apr;10(4):179-86. doi: 10.1016/j.molmed.2004.02.007. Trends Mol Med. 2004. PMID: 15059609 Review.
Cited by
-
Reduced Monocyte and Neutrophil Infiltration and Activation by P-Selectin/CD62P Inhibition Enhances Thrombus Resolution in Mice.Arterioscler Thromb Vasc Biol. 2024 Apr;44(4):954-968. doi: 10.1161/ATVBAHA.123.320016. Epub 2024 Feb 22. Arterioscler Thromb Vasc Biol. 2024. PMID: 38385292 Free PMC article.
-
From Classical Laboratory Parameters to Novel Biomarkers for the Diagnosis of Venous Thrombosis.Int J Mol Sci. 2020 Mar 11;21(6):1920. doi: 10.3390/ijms21061920. Int J Mol Sci. 2020. PMID: 32168924 Free PMC article. Review.
-
Tissue factor-bearing microparticles and thrombus formation.Arterioscler Thromb Vasc Biol. 2011 Apr;31(4):728-33. doi: 10.1161/ATVBAHA.109.200964. Epub 2011 Jan 20. Arterioscler Thromb Vasc Biol. 2011. PMID: 21252066 Free PMC article. Review.
-
Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice.Blood. 2017 Jul 13;130(2):181-191. doi: 10.1182/blood-2017-02-770479. Epub 2017 May 17. Blood. 2017. PMID: 28515093 Free PMC article.
-
SELP and SELPLG genetic variation is associated with cell surface measures of SELP and SELPLG: the Atherosclerosis Risk in Communities Carotid MRI Study.Clin Chem. 2009 Jun;55(6):1076-82. doi: 10.1373/clinchem.2008.119487. Epub 2009 Apr 24. Clin Chem. 2009. PMID: 19395438 Free PMC article.
References
-
- Wagner D D. Thromb Haemost. 1993;70:105–110. - PubMed
-
- Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Cell. 1993;74:541–554. - PubMed
-
- Palabrica T, Lobb R, Furie B C, Aronovitz M, Benjamin C, Hsu Y M, Sajer S A, Furie B. Nature (London) 1992;359:848–851. - PubMed
-
- Johnston G I, Bliss G A, Newman P J, McEver R P. J Biol Chem. 1990;265:21381–21385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

