Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit
- PMID: 11095744
- PMCID: PMC17675
- DOI: 10.1073/pnas.250478897
Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit
Erratum in
- Proc Natl Acad Sci U S A 2001 Aug 28;98(18):10515
Abstract
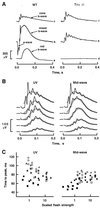
Retinal photoreceptors use the heterotrimeric G protein transducin to couple rhodopsin to a biochemical cascade that underlies the electrical photoresponse. Several isoforms of each transducin subunit are present in the retina. Although rods and cones seem to contain distinct transducin subunits, it is not known whether phototransduction in a given cell type depends strictly on a single form of each subunit. To approach this question, we have deleted the gene for the rod transducin alpha-subunit in mice. In hemizygous knockout mice, there was a small reduction in retinal transducin alpha-subunit content but retinal morphology and the physiology of single rods were largely normal. In homozygous knockout mice, a mild retinal degeneration occurred with age. Rod-driven components were absent from the electroretinogram, whereas cone-driven components were retained. Every photoreceptor examined by single-cell recording failed to respond to flashes, with one exception. The solitary responsive cell was insensitive, as expected for a cone, but had a rod-like spectral sensitivity and flash response kinetics that were slow, even for rods. These results indicate that most if not all rods use a single transducin type in phototransduction.
Figures


References
-
- Roof D, Makino C L. In: Principles and Practice of Ophthalmology. 2nd Ed. Alberts D, Jakobiec F, editors. Vol. 3. Philadelphia: Saunders; 2000. pp. 1624–1673.
-
- Lerea C L, Somers D E, Hurley J B, Klock I B, Bunt-Milam A H. Science. 1986;234:77–80. - PubMed
-
- Lerea C L, Bunt-Milam A H, Hurley J B. Neuron. 1989;3:367–376. - PubMed
-
- Fung B K-K, Lieberman B S, Lee R H. J Biol Chem. 1992;267:24782–24788. - PubMed
-
- Lee R H, Lieberman B S, Yamane H K, Bok D, Fung B K-K. J Biol Chem. 1992;267:24776–24781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

