What is a moment? "Cortical" sensory integration over a brief interval
- PMID: 11095747
- PMCID: PMC17676
- DOI: 10.1073/pnas.250483697
What is a moment? "Cortical" sensory integration over a brief interval
Abstract
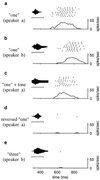
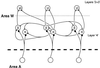
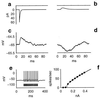
Recognition of complex temporal sequences is a general sensory problem that requires integration of information over time. We describe a very simple "organism" that performs this task, exemplified here by recognition of spoken monosyllables. The network's computation can be understood through the application of simple but generally unexploited principles describing neural activity. The organism is a network of very simple neurons and synapses; the experiments are simulations. The network's recognition capabilities are robust to variations across speakers, simple masking noises, and large variations in system parameters. The network principles underlying recognition of short temporal sequences are applied here to speech, but similar ideas can be applied to aspects of vision, touch, and olfaction. In this article, we describe only properties of the system that could be measured if it were a real biological organism. We delay publication of the principles behind the network's operation as an intellectual challenge: the essential principles of operation can be deduced based on the experimental results presented here alone. An interactive web site (http://neuron.princeton.edu/ approximately moment) is available to allow readers to design and carry out their own experiments on the organism.
Figures


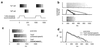
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

