DNA methylation and histone deacetylation in the control of gene expression: basic biochemistry to human development and disease
- PMID: 11097425
- PMCID: PMC5964960
- DOI: 10.3727/000000001783992731
DNA methylation and histone deacetylation in the control of gene expression: basic biochemistry to human development and disease
Abstract
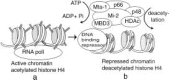
DNA methylation is a major determinant in the epigenetic silencing of genes. The mechanisms underlying the targeting of DNA methylation and the subsequent repression of transcription are relevant to human development and disease, as well as for attempts at somatic gene therapy. The success of transgenic technologies in plants and animals is also compromised by DNA methylation-dependent silencing pathways. Recent biochemical experiments provide a mechanistic foundation for understanding the influence of DNA methylation on transcription. The DNA methyltransferase Dnmt1, and several methyl-CpG binding proteins, MeCP2, MBD2, and MBD3, all associate with histone deacetylase. These observations firmly connect DNA methylation with chromatin modifications. They also provide new pathways for the potential targeting of DNA methylation to repressive chromatin as well as the assembly of repressive chromatin on methylated DNA. Here we discuss the implications of the methylation-acetylation connection for human cancers and the developmental syndromes Fragile X and Rett, which involve a mistargeting of DNA methylation-dependent repression.
Figures

Similar articles
-
Precipitous release of methyl-CpG binding protein 2 and histone deacetylase 1 from the methylated human multidrug resistance gene (MDR1) on activation.Mol Cell Biol. 2002 Mar;22(6):1844-57. doi: 10.1128/MCB.22.6.1844-1857.2002. Mol Cell Biol. 2002. PMID: 11865062 Free PMC article.
-
DNA methyltransferase 1 knock down induces gene expression by a mechanism independent of DNA methylation and histone deacetylation.J Biol Chem. 2004 Jul 2;279(27):27915-27. doi: 10.1074/jbc.M312823200. Epub 2004 Apr 15. J Biol Chem. 2004. PMID: 15087453
-
MBD2-MBD3 complex binds to hemi-methylated DNA and forms a complex containing DNMT1 at the replication foci in late S phase.Genes Cells. 2000 Aug;5(8):677-88. doi: 10.1046/j.1365-2443.2000.00359.x. Genes Cells. 2000. PMID: 10947852
-
Emerging connections between DNA methylation and histone acetylation.Cell Mol Life Sci. 2001 May;58(5-6):721-7. doi: 10.1007/pl00000895. Cell Mol Life Sci. 2001. PMID: 11437233 Free PMC article. Review.
-
DNA methylation and chromatin - unraveling the tangled web.Oncogene. 2002 Aug 12;21(35):5361-79. doi: 10.1038/sj.onc.1205609. Oncogene. 2002. PMID: 12154399 Review.
Cited by
-
Epigenetic mechanisms in cardiovascular complications of diabetes: towards future therapies.Mol Med. 2024 Sep 27;30(1):161. doi: 10.1186/s10020-024-00939-z. Mol Med. 2024. PMID: 39333854 Free PMC article. Review.
-
Valerian and valeric acid inhibit growth of breast cancer cells possibly by mediating epigenetic modifications.Sci Rep. 2021 Jan 28;11(1):2519. doi: 10.1038/s41598-021-81620-x. Sci Rep. 2021. PMID: 33510252 Free PMC article.
-
A new perspective on transcriptional system regulation (TSR): towards TSR profiling.PLoS One. 2008 Feb 20;3(2):e1656. doi: 10.1371/journal.pone.0001656. PLoS One. 2008. PMID: 18297136 Free PMC article.
-
Epigenetics in kidney diseases.Adv Clin Chem. 2021;104:233-297. doi: 10.1016/bs.acc.2020.09.005. Epub 2020 Oct 21. Adv Clin Chem. 2021. PMID: 34462056 Free PMC article. Review.
-
Transcriptional and epigenetic regulation of the integrin collagen receptor locus ITGA1-PELO-ITGA2.Biochim Biophys Acta. 2007 Sep-Oct;1769(9-10):546-58. doi: 10.1016/j.bbaexp.2007.06.004. Epub 2007 Jul 6. Biochim Biophys Acta. 2007. PMID: 17669516 Free PMC article.
References
-
- Almouzni G.; Wolffe A. P. Replication-coupled chromatin assembly is required for the repression of basal transcription in vivo. Genes Dev. 7:2033–2047; 1993. - PubMed
-
- Amir R. E.; Van den Veyver I. B.; Wan M.; Tran C. Q.; Francke U.; Zoghbi H. Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2 [see comments]. Nat. Genet. 23:185–188; 1999. - PubMed
-
- Antequera F.; Boyes J.; Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell 62:503–514; 1990. - PubMed
-
- Antequera F.; Macleod D.; Bird A. P. Specific protection of methylated CpGs in mammalian nuclei. Cell 58:509–517; 1989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
