Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture
- PMID: 11097910
- PMCID: PMC92464
- DOI: 10.1128/AEM.66.12.5329-5333.2000
Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture
Abstract
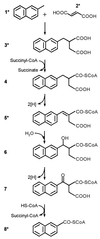
Anaerobic degradation of 2-methylnaphthalene was investigated with a sulfate-reducing enrichment culture. Metabolite analyses revealed two groups of degradation products. The first group comprised two succinic acid adducts which were identified as naphthyl-2-methyl-succinic acid and naphthyl-2-methylene-succinic acid by comparison with chemically synthesized reference compounds. Naphthyl-2-methyl-succinic acid accumulated to 0.5 microM in culture supernatants. Production of naphthyl-2-methyl-succinic acid was analyzed in enzyme assays with dense cell suspensions. The conversion of 2-methylnaphthalene to naphthyl-2-methyl-succinic acid was detected at a specific activity of 0.020 +/- 0.003 nmol min(-1) mg of protein(-1) only in the presence of cells and fumarate. We conclude that under anaerobic conditions 2-methylnaphthalene is activated by fumarate addition to the methyl group, as is the case in anaerobic toluene degradation. The second group of metabolites comprised 2-naphthoic acid and reduced 2-naphthoic acid derivatives, including 5,6,7,8-tetrahydro-2-naphthoic acid, octahydro-2-naphthoic acid, and decahydro-2-naphthoic acid. These compounds were also identified in an earlier study as products of anaerobic naphthalene degradation with the same enrichment culture. A pathway for anaerobic degradation of 2-methylnaphthalene analogous to that for anaerobic toluene degradation is proposed.
Figures

 , fatty acid methyl ester. Compounds marked with an asterisk are tentatively identified according to their mass spectra.
, fatty acid methyl ester. Compounds marked with an asterisk are tentatively identified according to their mass spectra.

References
-
- Bedessem M E, Swoboda-Colberg N G, Colberg P J S. Naphthalene mineralization coupled to sulfate-reduction in aquifer-derived enrichments. FEMS Microbiol Lett. 1997;152:213–218.
-
- Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

