Anion permeation in Ca(2+)-activated Cl(-) channels
- PMID: 11099350
- PMCID: PMC2231816
- DOI: 10.1085/jgp.116.6.825
Anion permeation in Ca(2+)-activated Cl(-) channels
Abstract
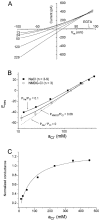
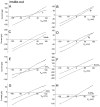
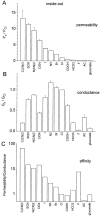
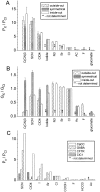
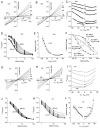
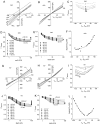
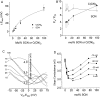
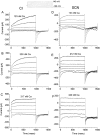
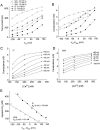
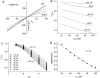
Ca(2+)-activated Cl channels (Cl(Ca)Cs) are an important class of anion channels that are opened by increases in cytosolic [Ca(2+)]. Here, we examine the mechanisms of anion permeation through Cl(Ca)Cs from Xenopus oocytes in excised inside-out and outside-out patches. Cl(Ca)Cs exhibited moderate selectivity for Cl over Na: P(Na)/P(Cl) = 0.1. The apparent affinity of Cl(Ca)Cs for Cl was low: K(d) = 73 mM. The channel had an estimated pore diameter >0.6 nm. The relative permeabilities measured under bi-ionic conditions by changes in E(rev) were as follows: C(CN)(3) > SCN > N(CN)(2) > ClO(4) > I > N(3) > Br > Cl > formate > HCO(3) > acetate = F > gluconate. The conductance sequence was as follows: N(3) > Br > Cl > N(CN)(2) > I > SCN > COOH > ClO(4) > acetate > HCO(3) = C(CN)(3) > gluconate. Permeant anions block in a voltage-dependent manner with the following affinities: C(CN)(3) > SCN = ClO(4) > N(CN)(2) > I > N(3) > Br > HCO(3) > Cl > gluconate > formate > acetate. Although these data suggest that anionic selectivity is determined by ionic hydration energy, other factors contribute, because the energy barrier for permeation is exponentially related to anion hydration energy. Cl(Ca)Cs exhibit weak anomalous mole fraction behavior, implying that the channel may be a multi-ion pore, but that ions interact weakly in the pore. The affinity of the channel for Ca(2+) depended on the permeant anion at low [Ca(2+)] (100-500 nM). Apparently, occupancy of the pore by a permeant anion increased the affinity of the channel for Ca(2+). The current was strongly dependent on pH. Increasing pH on the cytoplasmic side decreased the inward current, whereas increasing pH on the external side decreased the outward current. In both cases, the apparent pKa was voltage-dependent with apparent pKa at 0 mV = approximately 9.2. The channel may be blocked by OH(-) ions, or protons may titrate a site in the pore necessary for ion permeation. These data demonstrate that the permeation properties of Cl(Ca)Cs are different from those of CFTR or ClC-1, and provide insights into the nature of the Cl(Ca)C pore.
Figures



Similar articles
-
Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons.Proc Biol Sci. 1993 Jul 22;253(1336):69-75. doi: 10.1098/rspb.1993.0083. Proc Biol Sci. 1993. PMID: 7690484
-
Relationship between anion binding and anion permeability revealed by mutagenesis within the cystic fibrosis transmembrane conductance regulator chloride channel pore.J Physiol. 2001 Feb 15;531(Pt 1):51-66. doi: 10.1111/j.1469-7793.2001.0051j.x. J Physiol. 2001. PMID: 11179391 Free PMC article.
-
Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions.J Gen Physiol. 1998 May;111(5):653-65. doi: 10.1085/jgp.111.5.653. J Gen Physiol. 1998. PMID: 9565403 Free PMC article.
-
Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones.J Physiol. 1987 Apr;385:243-86. doi: 10.1113/jphysiol.1987.sp016493. J Physiol. 1987. PMID: 2443667 Free PMC article. Review.
-
Interactions of bromide, iodide, and fluoride with the pathways of chloride transport and diffusion in human neutrophils.J Gen Physiol. 1988 Jun;91(6):835-60. doi: 10.1085/jgp.91.6.835. J Gen Physiol. 1988. PMID: 3047312 Free PMC article. Review.
Cited by
-
Bicarbonate ion transport by the electrogenic Na+ /HCO3- cotransporter, NBCe1, is required for normal electrical slow-wave activity in mouse small intestine.Neurogastroenterol Motil. 2021 Sep;33(9):e14149. doi: 10.1111/nmo.14149. Epub 2021 Apr 10. Neurogastroenterol Motil. 2021. PMID: 33837991 Free PMC article.
-
Coupled movement of permeant and blocking ions in the CFTR chloride channel pore.J Physiol. 2003 Jun 1;549(Pt 2):375-85. doi: 10.1113/jphysiol.2002.038216. Epub 2003 Apr 4. J Physiol. 2003. PMID: 12679371 Free PMC article.
-
Calcium-dependent electrostatic control of anion access to the pore of the calcium-activated chloride channel TMEM16A.Elife. 2018 Oct 12;7:e39122. doi: 10.7554/eLife.39122. Elife. 2018. PMID: 30311910 Free PMC article.
-
Modes of Accessing Bicarbonate for the Regulation of Membrane Guanylate Cyclase (ROS-GC) in Retinal Rods and Cones.eNeuro. 2019 Feb 15;6(1):ENEURO.0393-18.2019. doi: 10.1523/ENEURO.0393-18.2019. eCollection 2019 Jan-Feb. eNeuro. 2019. PMID: 30783616 Free PMC article.
-
Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop.Proc Natl Acad Sci U S A. 2011 May 24;108(21):8891-6. doi: 10.1073/pnas.1102147108. Epub 2011 May 9. Proc Natl Acad Sci U S A. 2011. PMID: 21555582 Free PMC article.
References
-
- Begenisich T., Melvin J.E. Regulation of chloride channels in secretory epithelia. J. Membr. Biol. 1998;163:77–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

