Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement
- PMID: 11101510
- PMCID: PMC305872
- DOI: 10.1093/emboj/19.23.6371
Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement
Abstract
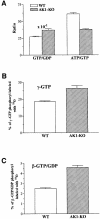
Efficient cellular energy homeostasis is a critical determinant of muscle performance, providing evolutionary advantages responsible for species survival. Phosphotransfer reactions, which couple ATP production and utilization, are thought to play a central role in this process. Here, we provide evidence that genetic disruption of AK1-catalyzed ss-phosphoryl transfer in mice decreases the potential of myofibers to sustain nucleotide ratios despite up-regulation of high-energy phosphoryl flux through glycolytic, guanylate and creatine kinase phosphotransfer pathways. A maintained contractile performance of AK1-deficient muscles was associated with higher ATP turnover rate and larger amounts of ATP consumed per contraction. Metabolic stress further aggravated the energetic cost in AK1(-/-) muscles. Thus, AK1-catalyzed phosphotransfer is essential in the maintenance of cellular energetic economy, enabling skeletal muscle to perform at the lowest metabolic cost.
Figures
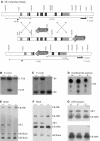
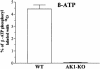
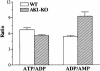

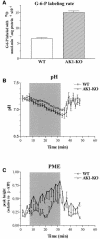


References
-
- Bandlow W., Strobel,G., Zoglowek,C., Oechsner,U. and Magdolen,V. (1988) Yeast adenylate kinase is active simultaneously in mitochondria and cytoplasm and is required for non-fermentative growth. Eur. J. Biochem., 178, 451–457. - PubMed
-
- Bessman S.P. and Carpenter,C.L. (1985) The creatine–creatine phosphate energy shuttle. Annu. Rev. Biochem., 54, 831–862. - PubMed
-
- Collavin L., Lazarevic,D., Utrera,R., Marzinotto,S., Monte,M. and Schneider,C. (1999) wt p53 dependent expression of a membrane-associated isoform of adenylate kinase. Oncogene, 18, 5879–5888. - PubMed
-
- de Haan A., Jones,D.A. and Sargeant,A.J. (1989) Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Pflugers Arch., 413, 422–428. - PubMed
-
- Dzeja P.P. and Terzic,A. (1998) Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J., 12, 523–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

