Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules
- PMID: 11101519
- PMCID: PMC305877
- DOI: 10.1093/emboj/19.23.6465
Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules
Abstract
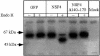
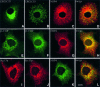
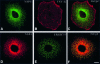
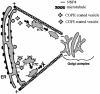
Membrane trafficking from the endoplasmic reticulum (ER) to the Golgi complex is mediated by pleiomorphic carrier vesicles that are driven along microtubule tracks by the action of motor proteins. Here we describe how NSP4, a rotavirus membrane glycoprotein, binds to microtubules and blocks ER-to-Golgi trafficking in vivo. NSP4 accumulates in a post-ER, microtubule-associated membrane compartment and prevents targeting of vesicular stomatitis virus glycoprotein (VSV-G) at a pre-Golgi step. NSP4 also redistributes beta-COP and ERGIC53, markers of a vesicular compartment that dynamically cycles between the ER and Golgi, to structures aligned along linear tracks radiating throughout the cytoplasm. This block in membrane trafficking is released when microtubules are depolymerized with nocodazole, indicating that vesicles containing NSP4 are tethered to the microtubule cytoskeleton. Disruption of microtubule-mediated membrane transport by a viral glycoprotein may represent a novel pathogenic mechanism and provides a new experimental tool for the dissection of early steps in exocytic transport.
Figures
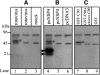


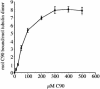
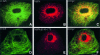
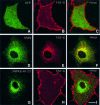
References
-
- Andersson H., Kappeler,F. and Hauri,H.P. (1999) Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J. Biol. Chem., 274, 15080–15084. - PubMed
-
- Ball J.M., Tian,P., Zheng,C.Q., Morris,A.P. and Estes,M.K. (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science, 272, 101–104. - PubMed
-
- Bannykh S.I., Nishimura,N. and Balch,W.E. (1998) Getting into the Golgi. Trends Cell Biol., 8, 21–25. - PubMed
-
- Barlowe C. (1998) COPII and selective export from the endoplasmic reticulum. Biochim. Biophys. Acta, 1404, 67–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

