A novel assembly mechanism for the DNA polymerase III holoenzyme DnaX complex: association of deltadelta' with DnaX(4) forms DnaX(3)deltadelta'
- PMID: 11101526
- PMCID: PMC305859
- DOI: 10.1093/emboj/19.23.6536
A novel assembly mechanism for the DNA polymerase III holoenzyme DnaX complex: association of deltadelta' with DnaX(4) forms DnaX(3)deltadelta'
Abstract
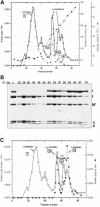
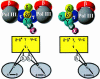
We have constructed a plasmid-borne artificial operon that expresses the six subunits of the DnaX complex of Escherichia coli DNA polymerase III holoenzyme: tau, gamma, delta, delta', chi and psi. Induction of this operon followed by assembly in vivo produced two taugamma mixed DnaX complexes with stoichiometries of tau(1)gamma(2)deltadelta'chipsi and tau(2)gamma(1)deltadelta'chipsi rather than the expected gamma(2)tau(2)deltadelta'chipsi. We observed the same heterogeneity when taugamma mixed DnaX complexes were reconstituted in vitro. Re-examination of homomeric DnaX tau and gamma complexes assembled either in vitro or in vivo also revealed a stoichiometry of DnaX(3)deltadelta'chipsi. Equilibrium sedimentation analysis showed that free DnaX is a tetramer in equilibrium with a free monomer. An assembly mechanism, in which the association of heterologous subunits with a homomeric complex alters the stoichiometry of the homomeric assembly, is without precedent. The significance of our findings to the architecture of the holoenzyme and the clamp-assembly apparatus of all other organisms is discussed.
Figures



Similar articles
-
Tau binds and organizes Escherichia coli replication proteins through distinct domains. Domain III, shared by gamma and tau, binds delta delta ' and chi psi.J Biol Chem. 2001 Feb 9;276(6):4447-53. doi: 10.1074/jbc.M009827200. Epub 2000 Nov 14. J Biol Chem. 2001. PMID: 11078742
-
Assembly of DNA polymerase III holoenzyme: co-assembly of gamma and tau is inhibited by DnaX complex accessory proteins but stimulated by DNA polymerase III core.J Biol Chem. 2001 Sep 14;276(37):35217-22. doi: 10.1074/jbc.M102735200. Epub 2001 Jul 19. J Biol Chem. 2001. PMID: 11463784
-
tau binds and organizes Escherichia coli replication proteins through distinct domains: domain III, shared by gamma and tau, oligomerizes DnaX.J Biol Chem. 2001 Sep 21;276(38):35842-6. doi: 10.1074/jbc.M103719200. Epub 2001 Jul 19. J Biol Chem. 2001. PMID: 11463787
-
Clamp loader structure predicts the architecture of DNA polymerase III holoenzyme and RFC.Curr Biol. 2001 Nov 13;11(22):R935-46. doi: 10.1016/s0960-9822(01)00559-0. Curr Biol. 2001. PMID: 11719243 Review.
-
Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences.Mol Microbiol. 2003 Sep;49(5):1157-65. doi: 10.1046/j.1365-2958.2003.03645.x. Mol Microbiol. 2003. PMID: 12940977 Review.
Cited by
-
Compartmentalization of the replication fork by single-stranded DNA-binding protein regulates translesion synthesis.Nat Struct Mol Biol. 2022 Sep;29(9):932-941. doi: 10.1038/s41594-022-00827-2. Epub 2022 Sep 20. Nat Struct Mol Biol. 2022. PMID: 36127468 Free PMC article.
-
Identification of β Clamp-DNA Interaction Regions That Impair the Ability of E. coli to Tolerate Specific Classes of DNA Damage.PLoS One. 2016 Sep 29;11(9):e0163643. doi: 10.1371/journal.pone.0163643. eCollection 2016. PLoS One. 2016. PMID: 27685804 Free PMC article.
-
Interaction of the beta sliding clamp with MutS, ligase, and DNA polymerase I.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8376-80. doi: 10.1073/pnas.121009498. Proc Natl Acad Sci U S A. 2001. PMID: 11459978 Free PMC article. Review.
-
A temperature-sensitive mutation in the dnaE gene of Caulobacter crescentus that prevents initiation of DNA replication but not ongoing elongation of DNA.J Bacteriol. 2004 Feb;186(4):1205-12. doi: 10.1128/JB.186.4.1205-1212.2004. J Bacteriol. 2004. PMID: 14762018 Free PMC article.
-
The unstructured C-terminus of the tau subunit of Escherichia coli DNA polymerase III holoenzyme is the site of interaction with the alpha subunit.Nucleic Acids Res. 2007;35(9):2813-24. doi: 10.1093/nar/gkm079. Epub 2007 Mar 13. Nucleic Acids Res. 2007. PMID: 17355988 Free PMC article.
References
-
- Cantor C.R. and Schimmel,P.R. (1980) Biophysical Chemistry Part I: The Conformation of Biological Macromolecules. W.H.Freeman, New York, NY, p. 137.
-
- Dallmann H.G. and McHenry,C.S. (1995) DnaX complex of Escherichia coli DNA polymerase III holoenzyme. Physical characterization of the DnaX subunits and complexes. J. Biol. Chem., 270, 29563–29569. - PubMed
-
- Dallmann H.G., Thimmig,R.L. and McHenry,C.S. (1995) DnaX complex of Escherichia coli DNA polymerase III holoenzyme. Central role of τ in initiation complex assembly and in determining the functional asymmetry of holoenzyme. J. Biol. Chem., 270, 29555–29562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

