Dynamic associations of heterochromatin protein 1 with the nuclear envelope
- PMID: 11101528
- PMCID: PMC305850
- DOI: 10.1093/emboj/19.23.6558
Dynamic associations of heterochromatin protein 1 with the nuclear envelope
Abstract
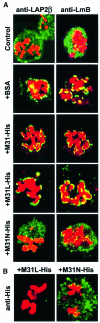
To study the dynamics of mammalian HP1 proteins we have microinjected recombinant forms of mHP1alpha, M31 and M32 into the cytoplasm of living cells. As could be expected from previous studies, the three fusion proteins were efficiently transported into the nucleus and targeted specific chromatin areas. However, before incorporation into these areas the exogenous proteins accumulated in a peripheral zone and associated closely with the nuclear envelope. This transient association did not occur when the cells were treated with deacetylase inhibitors, indicating an acetylation-inhibited interaction. In line with these observations, recombinant HP1 proteins exhibited saturable binding to purified nuclear envelopes and stained the nuclei of detergent-permeabilized cells in a rim-like fashion. Competition experiments with various M31 mutants allowed mapping of the nuclear envelope-binding site within an N-terminal region that includes the chromodomain. A His(6)-tagged peptide representing this region inhibited recruitment of LAP2beta and B-type lamins around the surfaces of condensed chromosomes, suggesting involvement of HP1 proteins in nuclear envelope reassembly.
Figures
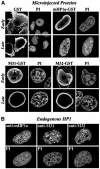
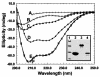
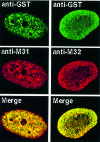

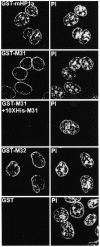



Similar articles
-
Nucleocytoplasmic sorting of macromolecules following mitosis: fate of nuclear constituents after inhibition of pore complex function.Eur J Cell Biol. 1989 Oct;50(1):209-19. Eur J Cell Biol. 1989. PMID: 2482180
-
Maintenance of stable heterochromatin domains by dynamic HP1 binding.Science. 2003 Jan 31;299(5607):721-5. doi: 10.1126/science.1078572. Science. 2003. PMID: 12560555
-
[Induction of nuclear envelope formation around individual chromosomes under impact of hypotonic shock].Tsitologiia. 2003;45(3):298-307. Tsitologiia. 2003. PMID: 14520887 Russian.
-
Nuclear envelope dynamics.Biochem Cell Biol. 2001;79(5):533-42. Biochem Cell Biol. 2001. PMID: 11716295 Review.
-
Review: lamina-associated polypeptide 2 isoforms and related proteins in cell cycle-dependent nuclear structure dynamics.J Struct Biol. 2000 Apr;129(2-3):335-45. doi: 10.1006/jsbi.2000.4212. J Struct Biol. 2000. PMID: 10806084 Review.
Cited by
-
Higher order chromatin organization in cancer.Semin Cancer Biol. 2013 Apr;23(2):109-15. doi: 10.1016/j.semcancer.2012.12.001. Epub 2012 Dec 22. Semin Cancer Biol. 2013. PMID: 23266653 Free PMC article. Review.
-
Recruitment of Tat to heterochromatin protein HP1 via interaction with CTIP2 inhibits human immunodeficiency virus type 1 replication in microglial cells.J Virol. 2003 May;77(9):5415-27. doi: 10.1128/jvi.77.9.5415-5427.2003. J Virol. 2003. PMID: 12692243 Free PMC article.
-
The Cell Biology of Heterochromatin.Cells. 2022 Apr 6;11(7):1247. doi: 10.3390/cells11071247. Cells. 2022. PMID: 35406810 Free PMC article. Review.
-
The coordination of nuclear envelope assembly and chromosome segregation in metazoans.Nucleus. 2020 Dec;11(1):35-52. doi: 10.1080/19491034.2020.1742064. Nucleus. 2020. PMID: 32208955 Free PMC article. Review.
-
Microtubule-interacting drugs induce moderate and reversible damage to human bone marrow mesenchymal stem cells.Cell Prolif. 2009 Aug;42(4):434-47. doi: 10.1111/j.1365-2184.2009.00607.x. Epub 2009 May 22. Cell Prolif. 2009. PMID: 19486015 Free PMC article.
References
-
- Bodoor K., Shaikh,S., Salina,D., Raharjo,W.H., Bastos,R., Lohka,M. and Burke,B. (1999) Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J. Cell Sci., 112, 2253–2264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

