Voltage-dependent conductance changes in the store-operated Ca2+ current ICRAC in rat basophilic leukaemia cells
- PMID: 11101641
- PMCID: PMC2270208
- DOI: 10.1111/j.1469-7793.2000.00295.x
Voltage-dependent conductance changes in the store-operated Ca2+ current ICRAC in rat basophilic leukaemia cells
Abstract
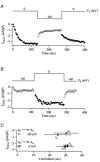
Tight-seal whole-cell patch-clamp experiments were carried out in order to investigate the effects of different holding potentials on the rate of development and amplitude of the Ca2+ release-activated Ca2+ current ICRAC in rat basophilic leukaemia (RBL-1) cells. ICRAC was monitored at -80 mV from fast voltage ramps, spanning 200 mV in 50 ms. At hyperpolarised potentials, the macroscopic CRAC conductance was lower than that seen at depolarised potentials. The conductance increased almost 5-fold over the voltage range -60 to +40 mV and was seen when the stores were depleted either by the combination of IP3 and thapsigargin in high Ca2+ buffer, or passively with 10 mM EGTA or BAPTA. The voltage-dependent conductance of the CRAC channels could not be fully accounted for by Ca2+-dependent fast inactivation, nor by other slower inhibitory mechanisms. It also did not seem to involve intracellular Mg2+ or the polycations spermine and spermidine. Voltage step relaxation experiments revealed that the voltage-dependent conductance changes developed and reversed slowly, with a time constant of several seconds at -60 mV. In the presence of physiological levels of intracellular Ca2+ buffers, ICRAC was barely detectable when cells were clamped at -60 mV and dialysed with IP3 and thapsigargin, but at 0 mV the current in low Ca2+ buffer was as large as that seen in high Ca2+ buffer. Our results suggest that CRAC channels exhibit slow voltage-dependent conductance changes which can triple the current amplitude over the physiological range of voltages normally encountered by these cells. The role of this conductance change and possible underlying mechanisms are discussed.
Figures




References
-
- Artalejo AR, Ellory JC, Parekh AB. Ca2+-dependent capacitance increases in rat basophilic leukaemia cells following activation of store-operated Ca2+ entry and dialysis with high Ca2+-containing intracellular solution. Pflügers Archiv. 1998;436:934–939. - PubMed
-
- Ficker E, Taglialatela M, Wible MA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. - PubMed
-
- Fierro L, Parekh AB. Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in Rbl-1 cells. Journal of Membrane Biology. 1999a;168:9–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

