Molecular diversity of the repolarizing voltage-gated K+ currents in mouse atrial cells
- PMID: 11101645
- PMCID: PMC2270194
- DOI: 10.1111/j.1469-7793.2000.00345.x
Molecular diversity of the repolarizing voltage-gated K+ currents in mouse atrial cells
Abstract
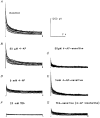
Voltage-clamp studies on atrial myocytes isolated from adult and postnatal day 15 (P15) C57BL6 mice demonstrate the presence of three kinetically distinct Ca2+-independent, depolarization-activated outward K+ currents: a fast, transient outward current (Ito,f), a rapidly activating, slowly inactivating current (IK,s) and a non-inactivating, steady-state current (Iss). The time- and voltage-dependent properties of to,f, IK,s and Iss in adult and P15 atrial cells are indistinguishable. Pharmacological experiments reveal the presence of two components of IK,s: one that is blocked selectively by 50 microM 4-aminopyridine (4-AP), and a 4-AP-insensitive component that is blocked by 25 mM TEA; Iss is also partially attenuated by 25 mM TEA. There are also two components of IK,s recovery from steady-state inactivation. To explore the molecular correlates of mouse atrial IK,s and Iss, whole-cell voltage-clamp recordings were obtained from P15 and adult atrial cells isolated from transgenic mice expressing a mutant Kv2.1 alpha subunit (Kv2.1N216Flag) that functions as a dominant negative, and from P15 atrial myocytes exposed to (1 microM) antisense oligodeoxynucleotides (AsODNs) targeted against Kv1.5 or Kv2.1. Peak outward K+ current densities are attenuated significantly in atrial myocytes isolated from P15 and adult Kv2.1N216Flag-expressing animals and in P15 cells exposed to AsODNs targeted against either Kv1.5 or Kv2.1. Analysis of the decay phases of the outward currents evoked during long (5 s) depolarizing voltage steps revealed that IK, s is selectively attenuated in cells exposed to the Kv1.5 AsODN, whereas both IK,s and Iss are attenuated in the presence of the Kv2. 1 AsODN. In P15 and adult Kv2.1N216Flag-expressing atrial cells, mean +/- s.e.m. IK,s and Iss densities are also significantly lower than in non-transgenic atrial cells. In addition, pharmacological experiments reveal that the TEA-sensitive component IK,s is selectively eliminated in P15 and adult Kv2.1N216Flag-expressing atrial cells. Taken together, the results presented here reveal that both Kv1.5 and Kv2.1 contribute to mouse atrial IK,s, consistent with the presence of two molecularly distinct components of IK,s. In addition, Kv2.1 contributes to mouse atrial Iss.
Figures




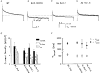


Similar articles
-
Molecular correlates of the calcium-independent, depolarization-activated K+ currents in rat atrial myocytes.J Physiol. 1999 Jun 1;517 ( Pt 2)(Pt 2):407-20. doi: 10.1111/j.1469-7793.1999.0407t.x. J Physiol. 1999. PMID: 10332091 Free PMC article.
-
Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes.J Physiol. 1999 Dec 15;521 Pt 3(Pt 3):587-99. doi: 10.1111/j.1469-7793.1999.00587.x. J Physiol. 1999. PMID: 10601491 Free PMC article.
-
Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes.J Gen Physiol. 1999 May;113(5):661-78. doi: 10.1085/jgp.113.5.661. J Gen Physiol. 1999. PMID: 10228181 Free PMC article.
-
Regulation of voltage-gated K+ channel expression in the developing mammalian myocardium.J Neurobiol. 1998 Oct;37(1):37-59. doi: 10.1002/(sici)1097-4695(199810)37:1<37::aid-neu4>3.0.co;2-9. J Neurobiol. 1998. PMID: 9777731 Review.
-
Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium.J Physiol. 2000 Jun 1;525 Pt 2(Pt 2):285-98. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. J Physiol. 2000. PMID: 10835033 Free PMC article. Review.
Cited by
-
Molecular Basis of Functional Myocardial Potassium Channel Diversity.Card Electrophysiol Clin. 2016 Jun;8(2):257-73. doi: 10.1016/j.ccep.2016.01.001. Epub 2016 Mar 24. Card Electrophysiol Clin. 2016. PMID: 27261820 Free PMC article. Review.
-
Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death.Circ Res. 2015 Jun 5;116(12):1971-88. doi: 10.1161/CIRCRESAHA.116.305017. Circ Res. 2015. PMID: 26044251 Free PMC article. Review.
-
Species-Dependent Mechanisms of Cardiac Arrhythmia: A Cellular Focus.Clin Med Insights Cardiol. 2017 Feb 2;11:1179546816686061. doi: 10.1177/1179546816686061. eCollection 2017. Clin Med Insights Cardiol. 2017. PMID: 28469490 Free PMC article. Review.
-
Kcne4 deletion sex- and age-specifically impairs cardiac repolarization in mice.FASEB J. 2016 Jan;30(1):360-9. doi: 10.1096/fj.15-278754. Epub 2015 Sep 23. FASEB J. 2016. PMID: 26399785 Free PMC article.
-
SUMOylation determines the voltage required to activate cardiac IKs channels.Proc Natl Acad Sci U S A. 2017 Aug 8;114(32):E6686-E6694. doi: 10.1073/pnas.1706267114. Epub 2017 Jul 25. Proc Natl Acad Sci U S A. 2017. PMID: 28743749 Free PMC article.
References
-
- Anumonwo JMB, Freeman LC, Kwok WM, Kass RS. Potassium channels in the heart: electrophysiology and pharmacological regulation. Cardiovascular Drug Reviews. 1991;9:299–316.
-
- Barry DM, Nerbonne JM. Myocardial potassium channels: Electrophysiological and molecular diversity. Annual Review of Physiology. 1996;58:363–394. - PubMed
-
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 α subunit. Circulation Research. 1998;83:560–567. - PubMed
-
- Deal KK, England SK, Tamkun MM. Molecular physiology of cardiac potassium channels. Physiological Reviews. 1996;76:49–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

