Accommodation to depolarizing and hyperpolarizing currents in cutaneous afferents of the human median and sural nerves
- PMID: 11101656
- PMCID: PMC2270209
- DOI: 10.1111/j.1469-7793.2000.00483.x
Accommodation to depolarizing and hyperpolarizing currents in cutaneous afferents of the human median and sural nerves
Abstract
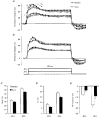
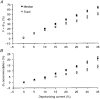
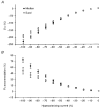
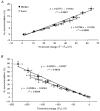
To determine whether accommodation to depolarizing and hyperpolarizing stimuli differs for cutaneous afferents in the median and sural nerves, studies were performed in normal human subjects using threshold electrotonus. The changes in threshold for compound sensory action potentials of 50 % of maximum were recorded when the nerves were subjected to long-lasting depolarizing and hyperpolarizing DC. The premise was that the threshold changes largely mirror the underlying electrotonic changes in membrane potential. The maximal threshold changes produced by depolarizing and hyperpolarizing currents were greater for median afferents, suggesting that the DC produced greater changes in membrane potential in these afferents. Median afferents underwent greater accommodation to depolarizing currents than sural afferents and a greater threshold undershoot at the end of the currents, suggesting greater activity of a slow K+ conductance. Median afferents also underwent greater accommodation to hyperpolarizing currents, suggesting greater inward rectification. These conductances are voltage dependent, and the differences in accommodation could be due to greater changes in membrane potential for the median nerve. The changes in threshold produced by long-lasting depolarizing and hyperpolarizing currents of graded intensity were therefore measured. When the threshold changes were matched for the two nerves, median afferents underwent 22.4 % more accommodation to depolarizing currents and 28.7 % more accommodation to hyperpolarizing currents. We conclude that there is greater expression of two internodally located conductances responsible for accommodation on median afferents. The biophysical differences identified in this study might contribute to the finding that sural afferents have a greater tendency to dysfunction than median afferents.
Figures

References
-
- Birch BD, Kocsis JD, Di Gregorio F, Bhisitkul RB, Waxman SG. A voltage- and time-dependent rectification in rat dorsal spinal root axons. Journal of Neurophysiology. 1991;66:719–728. - PubMed
-
- Bostock H. Mechanisms of accommodation and adaptation in myelinated axons. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon. New York: Oxford University Press; 1995. pp. 311–327.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

