Zinc inhibits miniature GABAergic currents by allosteric modulation of GABAA receptor gating
- PMID: 11102466
- PMCID: PMC6773059
- DOI: 10.1523/JNEUROSCI.20-23-08618.2000
Zinc inhibits miniature GABAergic currents by allosteric modulation of GABAA receptor gating
Abstract
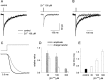
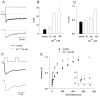
Zinc is abundantly present in the CNS, and after nerve stimulation is thought to be released in sufficient quantity to modulate the synaptic transmission. Although it is known that this divalent cation inhibits the GABAergic synaptic currents, the underlying mechanisms were not fully elucidated. Here we report that zinc reduced the amplitude, slowed the rise time, and accelerated the decay of mIPSCs in cultured hippocampal neurons. The analysis of current responses to rapid GABA applications and model simulations indicated that these effects on mIPSCs are caused by zinc modulation of GABA(A) receptor gating. In particular, zinc slowed the onset of GABA-evoked currents by decreasing both the binding (k(on)) and the transition rate from closed to open state (beta(2)). Moreover, slower onset and recovery from desensitization as well as an increased unbinding rate (k(off)) were shown to underlie the accelerated deactivation kinetics in the presence of zinc. The nonequilibrium conditions of GABA(A) receptor activation were found to strongly affect zinc modulation of this receptor. In particular, an extremely fast clearance of synaptic GABA is implicated to be responsible for a stronger zinc effect on mIPSCs than on current responses to exogenous GABA. Finally, the analysis of currents evoked by GABA coapplied with zinc indicated that the interaction between zinc and GABA(A) receptors was too slow to explain zinc effects in terms of competitive antagonism. In conclusion, our results provide evidence that inhibition of mIPSCs by zinc is attributable to the allosteric modulation of GABA(A) receptor gating.
Figures





References
-
- Assaf SY, Chung SH. Release of endogenous Zn++ from brain tissue during activity. Nature. 1984;308:734–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
