Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin
- PMID: 11102480
- PMCID: PMC6773047
- DOI: 10.1523/JNEUROSCI.20-23-08736.2000
Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin
Abstract
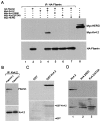
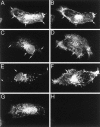
Kv4.2 potassium channels play a critical role in postsynaptic excitability. Immunocytochemical studies reveal a somatodendritic Kv4.2 expression pattern, with the channels concentrated mainly at dendritic spines. The molecular mechanism that underlies the localization of Kv4.2 to this subcellular region is unknown. We used the yeast two-hybrid system to identify the Kv4.2-associated proteins that are involved in channel localization. Here we demonstrate a direct interaction between Kv4.2 and the actin-binding protein, filamin. We show that Kv4.2 and filamin can be coimmunoprecipitated both in vitro and in brain and that Kv4.2 and filamin share an overlapping expression pattern in the cerebellum and cultured hippocampal neurons. To examine the functional consequences of this interaction, we expressed Kv4.2 in filamin(+) and filamin(-) cells and performed immunocytochemical and electrophysiological analyses. Our results indicate that Kv4.2 colocalizes with filamin at filopodial roots in filamin(+) cells but shows a nonspecific expression pattern in filamin(-) cells, with no localization to filopodial roots. Furthermore, the magnitude of whole-cell Kv4.2 current density is approximately 2.7-fold larger in filamin(+) cells as compared with these currents in filamin(-) cells. We propose that filamin may function as a scaffold protein in the postsynaptic density, mediating a direct link between Kv4.2 and the actin cytoskeleton, and that this interaction is essential for the generation of appropriate Kv4.2 current densities.
Figures





Similar articles
-
Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding.Mol Pharmacol. 2007 Sep;72(3):622-30. doi: 10.1124/mol.107.038026. Epub 2007 Jun 22. Mol Pharmacol. 2007. PMID: 17586600
-
Direct interaction between the actin-binding protein filamin-A and the inwardly rectifying potassium channel, Kir2.1.J Biol Chem. 2003 Oct 24;278(43):41988-97. doi: 10.1074/jbc.M307479200. Epub 2003 Aug 14. J Biol Chem. 2003. PMID: 12923176
-
Interaction of the pacemaker channel HCN1 with filamin A.J Biol Chem. 2004 Oct 15;279(42):43847-53. doi: 10.1074/jbc.M401598200. Epub 2004 Jul 30. J Biol Chem. 2004. PMID: 15292205
-
Structural and functional aspects of filamins.Biochim Biophys Acta. 2001 Apr 23;1538(2-3):99-117. doi: 10.1016/s0167-4889(01)00072-6. Biochim Biophys Acta. 2001. PMID: 11336782 Review.
-
Structural portrait of filamin interaction mechanisms.Curr Protein Pept Sci. 2010 Nov;11(7):639-50. doi: 10.2174/138920310794109111. Curr Protein Pept Sci. 2010. PMID: 20887254 Review.
Cited by
-
Filamin a binds to CCR2B and regulates its internalization.PLoS One. 2010 Aug 17;5(8):e12212. doi: 10.1371/journal.pone.0012212. PLoS One. 2010. PMID: 20808917 Free PMC article.
-
New insights into the roles of Xin repeat-containing proteins in cardiac development, function, and disease.Int Rev Cell Mol Biol. 2014;310:89-128. doi: 10.1016/B978-0-12-800180-6.00003-7. Int Rev Cell Mol Biol. 2014. PMID: 24725425 Free PMC article. Review.
-
Neuritin Up-regulates Kv4.2 α-Subunit of Potassium Channel Expression and Affects Neuronal Excitability by Regulating the Calcium-Calcineurin-NFATc4 Signaling Pathway.J Biol Chem. 2016 Aug 12;291(33):17369-81. doi: 10.1074/jbc.M115.708883. Epub 2016 Jun 15. J Biol Chem. 2016. PMID: 27307045 Free PMC article.
-
Distinct regional and subcellular localization of the actin-binding protein filamin A in the mature rat brain.J Comp Neurol. 2012 Sep 1;520(13):3013-34. doi: 10.1002/cne.23106. J Comp Neurol. 2012. PMID: 22434607 Free PMC article.
-
Mechanical response of single filamin A (ABP-280) molecules and its role in the actin cytoskeleton.J Muscle Res Cell Motil. 2002;23(5-6):525-34. doi: 10.1023/a:1023418725001. J Muscle Res Cell Motil. 2002. PMID: 12785102 Review.
References
-
- Alonso G, Widmer H. Clustering of Kv4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
-
- Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–157. - PubMed
-
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Circ Res. 1995;77:361–369. - PubMed
-
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
