Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy
- PMID: 11102487
- PMCID: PMC6773070
- DOI: 10.1523/JNEUROSCI.20-23-08788.2000
Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy
Abstract
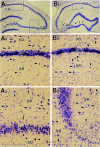
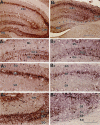
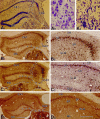
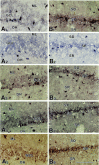
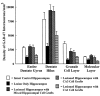
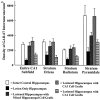
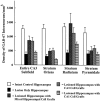
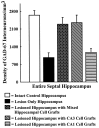
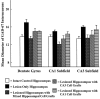
Degeneration of CA3-pyramidal neurons in hippocampus after intracerebroventricular kainic acid (KA) administration, a model of temporal lobe epilepsy, results in hyperexcitability within both dentate gyrus and the CA1 subfield. It also leads to persistent reductions in hippocampal glutamate decarboxylase (GAD) interneuron numbers without diminution in Nissl-stained interneuron numbers, indicating loss of GAD expression in a majority of interneurons. We hypothesize that enduring loss of GAD expression in hippocampal interneurons after intracerebroventricular KA is attributable to degeneration of their CA3 afferent input; therefore, fetal CA3 grafts can restore GAD interneuron numbers through graft axon reinnervation of the host. We analyzed GAD interneuron density in the adult rat hippocampus at 6 months after KA administration after grafting of fetal mixed hippocampal, CA3 or CA1 cells into the CA3 region at 45 d after lesion, in comparison with "lesion-only" and intact hippocampus. In dentate and CA1 regions of the lesioned hippocampus receiving grafts of either mixed hippocampal or CA3 cells, GAD interneuron density was both significantly greater than lesion-only hippocampus and comparable with the intact hippocampus. In the CA3 region, GAD interneuron density was significantly greater than lesion-only hippocampus but less than the intact hippocampus. Collectively, the overall GAD interneuron density in the lesioned hippocampus receiving either mixed hippocampal or CA3 grafts was restored to that in the intact hippocampus. In contrast, GADinterneuron density in the lesioned hippocampus receiving CA1 grafts remained comparable with lesion-only hippocampus. Thus, grafts containing CA3 cells restore CA3 lesion-induced depletions in hippocampal GAD interneurons, likely by reinnervation of GAD-deficient interneurons. This specific graft-mediated effect is beneficial because reactivation of interneurons could ameliorate both loss of functional inhibition and hyperexcitability in CA3-lesioned hippocampus.
Figures

Similar articles
-
Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons.Exp Neurol. 2001 Jun;169(2):276-97. doi: 10.1006/exnr.2001.7668. Exp Neurol. 2001. PMID: 11358442
-
Restoration of calbindin after fetal hippocampal CA3 cell grafting into the injured hippocampus in a rat model of temporal lobe epilepsy.Hippocampus. 2007;17(10):943-56. doi: 10.1002/hipo.20311. Hippocampus. 2007. PMID: 17604349 Free PMC article.
-
Fetal hippocampal CA3 cell grafts transplanted to lesioned CA3 region of the adult hippocampus exhibit long-term survival in a rat model of temporal lobe epilepsy.Neurobiol Dis. 2001 Dec;8(6):942-52. doi: 10.1006/nbdi.2001.0440. Neurobiol Dis. 2001. PMID: 11741390
-
Interneurons in rat hippocampus after cerebral ischemia. Morphometric, functional, and therapeutic investigations.Acta Neurol Scand Suppl. 1993;150:1-32. Acta Neurol Scand Suppl. 1993. PMID: 7907456 Review.
-
Development of fetal hippocampal grafts in intact and lesioned hippocampus.Prog Neurobiol. 1996 Dec;50(5-6):597-653. doi: 10.1016/s0301-0082(96)00048-2. Prog Neurobiol. 1996. PMID: 9015829 Review.
Cited by
-
Effects of Swimming Exercise on Limbic and Motor Cortex Neurogenesis in the Kainate-Lesion Model of Temporal Lobe Epilepsy.Cardiovasc Psychiatry Neurol. 2016;2016:3915767. doi: 10.1155/2016/3915767. Epub 2016 May 22. Cardiovasc Psychiatry Neurol. 2016. PMID: 27313873 Free PMC article.
-
Grafted hPSC-derived GABA-ergic interneurons regulate seizures and specific cognitive function in temporal lobe epilepsy.NPJ Regen Med. 2022 Aug 1;7(1):38. doi: 10.1038/s41536-022-00234-7. NPJ Regen Med. 2022. PMID: 35915118 Free PMC article.
-
Is exposure to enriched environment beneficial for functional post-lesional recovery in temporal lobe epilepsy?Neurosci Biobehav Rev. 2008;32(4):657-74. doi: 10.1016/j.neubiorev.2007.10.004. Epub 2007 Nov 28. Neurosci Biobehav Rev. 2008. PMID: 18178250 Free PMC article. Review.
-
Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy.Stem Cells. 2010 Jul;28(7):1153-64. doi: 10.1002/stem.446. Stem Cells. 2010. PMID: 20506409 Free PMC article.
-
Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy.Epilepsia. 2008 Jun;49 Suppl 5(0 5):26-41. doi: 10.1111/j.1528-1167.2008.01635.x. Epilepsia. 2008. PMID: 18522598 Free PMC article. Review.
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–246. - PubMed
-
- Bernard C, Esclapez M, Hirsch JC, Ben-Ari Y. Interneurons are not so dormant in temporal lobe epilepsy: a critical reappraisal of the dormant basket cell hypothesis. Epilepsy Res. 1998;32:93–103. - PubMed
-
- Best N, Mitchell J, Baimbridge KG, Wheal HV. Changes in parvalbumin immunoreactive neurons in the rat hippocampus following a kainic acid lesion. Neurosci Lett. 1993;155:1–6. - PubMed
-
- Buzsaki G. Feed-forward inhibition in the hippocampal formation. Prog Neurobiol. 1984;22:131–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
