NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro
- PMID: 11102491
- PMCID: PMC6773069
- DOI: 10.1523/JNEUROSCI.20-23-08831.2000
NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro
Abstract
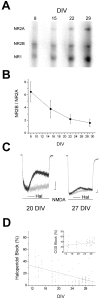
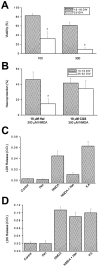
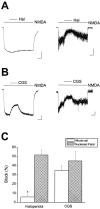
The development of cortical neurons in vivo and in vitro is accompanied by alterations in NMDA receptor subunit expression and concomitant modifications in the pharmacological profile of NMDA-activated ionic currents. For example, we observed that with decreasing NR2B/NR2A subunit expression ratio, the block of NMDA receptor-mediated whole-cell responses by the NR2B-selective antagonist haloperidol was also decreased. In mature cultures (>22 d in vitro), however, NMDA responses obtained from excised nucleated macropatches, which comprised a large portion of the soma, remained strongly antagonized by haloperidol. These results suggest that in more mature neurons NR1/NR2B receptors appear to be preferentially expressed in the cell body. As predicted from the whole-cell recording pharmacological profile, NMDA-induced toxicity was largely unaffected by haloperidol in mature cultures. However, haloperidol effectively blocked glutamate toxicity in the same cultures, suggesting that the neurotoxic actions of this amino acid were mostly due to the activation of somatic NMDA receptors. In experiments in which the potency of glutamate toxicity was increased by the transport inhibitor l-trans-pyrrolidine-2,4-dicarboxylic acid, the neuroprotective effects of haloperidol were significantly diminished. This was likely because of the fact that glutamate, now toxic at much lower concentrations, was able to reach and activate dendritic receptors under these conditions. These results strongly argue that exogenous glutamate and NMDA normally induce excitotoxicity at distinct cellular locations in mature mixed neuronal cultures and that NR1/NR2B receptors remain an important component in the expression of glutamate, but not NMDA-induced excitotoxicity.
Figures


References
-
- Aizenman E, Hartnett KA. The action of CGS-19755 on the redox enhancement of NMDA toxicity in rat cortical neurons in vitro. Brain Res. 1992;585:28–34. - PubMed
-
- Blitzblau R, Gupta S, Djali S, Robinson MB, Rosenberg PA. The glutamate transport inhibitor l-trans-pyrrolidine-2,4-dicarboxylate indirectly evokes NMDA receptor mediated neurotoxicity in rat cortical cultures. Eur J Neurosci. 1996;8:1840–1852. - PubMed
-
- Boeckman FA, Aizenman E. Pharmacological properties of acquired excitotoxicity in Chinese hamster ovary cells transfected with N-methyl-d-aspartate receptor subunits. J Pharmacol Exp Ther. 1996;279:515–523. - PubMed
-
- Brimecombe JC, Gallagher MJ, Lynch DR, Aizenman E. An NR2B point mutation affecting haloperidol and CP101,606 sensitivity of single recombinant N-methyl-d-aspartate receptors. J Pharmacol Exp Ther. 1998;286:627–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
