Reliability of a fly motion-sensitive neuron depends on stimulus parameters
- PMID: 11102498
- PMCID: PMC6773076
- DOI: 10.1523/JNEUROSCI.20-23-08886.2000
Reliability of a fly motion-sensitive neuron depends on stimulus parameters
Abstract
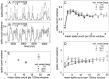
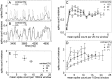
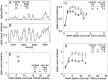
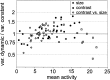
The variability of responses of sensory neurons constrains how reliably animals can respond to stimuli in the outside world. We show for a motion-sensitive visual interneuron of the fly that the variability of spike trains depends on the properties of the motion stimulus, although differently for different stimulus parameters. (1) The spike count variances of responses to constant and to dynamic stimuli lie in the same range. (2) With increasing stimulus size, the variance may slightly decrease. (3) Increasing pattern contrast reduces the variance considerably. For all stimulus conditions, the spike count variance is much smaller than the mean spike count and does not depend much on the mean activity apart from very low activities. Using a model of spike generation, we analyzed how the spike count variance depends on the membrane potential noise and the deterministic membrane potential fluctuations at the spike initiation zone of the neuron. In a physiologically plausible range, the variance is affected only weakly by changes in the dynamics or the amplitude of the deterministic membrane potential fluctuations. In contrast, the amplitude and dynamics of the membrane potential noise strongly influence the spike count variance. The membrane potential noise underlying the variability of the spike responses in the motion-sensitive neuron is concluded to be affected considerably by the contrast of the stimulus but by neither its dynamics nor its size.
Figures





References
-
- Barberini CL, Horwitz GD, Newsome WT. A comparison of spiking statistics in motion sensing neurons of flies and monkeys. In: Zanker JM, Zeil J, editors. Computational, neural and ecological constraints of visual motion processing. Springer; Berlin: 2000. pp. 307–320.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
