The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory
- PMID: 11102506
- PMCID: PMC6773086
- DOI: 10.1523/JNEUROSCI.20-23-08954.2000
The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory
Abstract
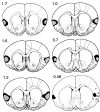
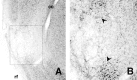
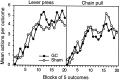
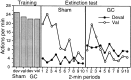
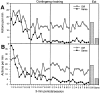
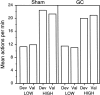
In three experiments, we assessed the effect of lesions aimed at the gustatory region of the insular cortex on instrumental conditioning in rats. In experiment 1, the lesion had no effect on the acquisition of either lever pressing or chain pulling in food-deprived rats whether these actions earned food pellets or a maltodextrin solution. The lesion did, however, attenuate the impact of outcome devaluation, induced by sensory-specific satiety, on instrumental performance but only when assessed in an extinction test. This effect was not secondary to an impairment in instrumental learning; in experiment 2, no evidence was found to suggest that the lesioned rats differed from shams in their ability to encode the specific action-outcome contingencies to which they were exposed during training. In experiment 3, however, lesioned rats were found to be insensitive to the impact of an incentive learning treatment conducted when they were undeprived; although, again, this deficit was confined to a test conducted in extinction. These results are consistent with the view that, in instrumental conditioning, the gustatory region of the insular cortex is involved in encoding the taste of food outcomes in memory and, hence, in encoding the incentive value assigned to these outcomes on the basis of prevailing motivational conditions.
Figures



References
-
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta). Physiol Psychol. 1981;95:961–977. - PubMed
-
- Balleine BW. The role of incentive learning in instrumental performance following shifts in primary motivation. J Exp Psychol Anim Behav Process. 1992;18:236–250. - PubMed
-
- Balleine BW. Incentive processes in instrumental conditioning. In: Mowrer R, Klein S, editors. Handbook of contemporary learning theories. Erlbaum; Hillsdale, NJ: 2000. pp. 307–366.
-
- Balleine BW, Dickinson A. Role of cholecystokinin in the motivational control of instrumental action in rats. Behav Neurosci. 1994;108:590–605. - PubMed
-
- Balleine BW, Dickinson A. The role of incentive learning in instrumental outcome revaluation by sensory-specific satiety. Anim Learn Behav. 1998;26:46–59.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
