Distinct FTDP-17 missense mutations in tau produce tau aggregates and other pathological phenotypes in transfected CHO cells
- PMID: 11102510
- PMCID: PMC15059
- DOI: 10.1091/mbc.11.12.4093
Distinct FTDP-17 missense mutations in tau produce tau aggregates and other pathological phenotypes in transfected CHO cells
Abstract
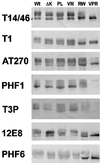
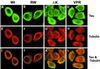
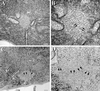
Multiple tau gene mutations are pathogenic for hereditary frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), with filamentous tau aggregates as the major lesions in the CNS of these patients. Recent studies have shown that bacterially expressed recombinant tau proteins with FTDP-17 missense mutations cause functional impairments, i.e., a reduced ability of mutant tau to bind to or promote the assembly of microtubules. To investigate the biological consequences of FTDP-17 tau mutants and assess their ability to form filamentous aggregates, we engineered Chinese hamster ovary cell lines to stably express tau harboring one or several different FTDP-17 mutations and showed that different tau mutants produced distinct pathological phenotypes. For example, delta K, but not several other single tau mutants (e.g., V337 M, P301L, R406W), developed insoluble amorphous and fibrillar aggregates, whereas a triple tau mutant (VPR) containing V337M, P301L, and R406W substitutions also formed similar aggregates. Furthermore, the aggregates increased in size over time in culture. Significantly, the formation of aggregated delta K and VPR tau protein correlated with reduced affinity of these mutants to bind microtubules. Reduced phosphorylation and altered proteolysis was also observed in R406W and delta K tau mutants. Thus, distinct pathological phenotypes, including the formation of insoluble filamentous tau aggregates, result from the expression of different FTDP-17 tau mutants in transfected Chinese hamster ovary cells and implies that these missense mutations cause diverse neurodegenerative FTDP-17 syndromes by multiple mechanisms.
Figures


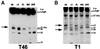
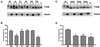

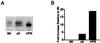
Similar articles
-
Missense tau mutations identified in FTDP-17 have a small effect on tau-microtubule interactions.Brain Res. 2000 Jan 17;853(1):5-14. doi: 10.1016/s0006-8993(99)02124-1. Brain Res. 2000. PMID: 10627302
-
Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17.Science. 1998 Dec 4;282(5395):1914-7. doi: 10.1126/science.282.5395.1914. Science. 1998. PMID: 9836646
-
Missense point mutations of tau to segregate with FTDP-17 exhibit site-specific effects on microtubule structure in COS cells: a novel action of R406W mutation.J Neurosci Res. 2000 May 1;60(3):380-7. doi: 10.1002/(SICI)1097-4547(20000501)60:3<380::AID-JNR13>3.0.CO;2-5. J Neurosci Res. 2000. PMID: 10797541
-
Fibrillogenesis of tau: insights from tau missense mutations in FTDP-17.Brain Pathol. 1999 Oct;9(4):695-705. doi: 10.1111/j.1750-3639.1999.tb00551.x. Brain Pathol. 1999. PMID: 10517508 Free PMC article. Review.
-
Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer's disease.Biochim Biophys Acta. 2000 Jul 26;1502(1):110-21. doi: 10.1016/s0925-4439(00)00037-5. Biochim Biophys Acta. 2000. PMID: 10899436 Review.
Cited by
-
Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation.Nat Chem Biol. 2012 Feb 26;8(4):393-9. doi: 10.1038/nchembio.797. Nat Chem Biol. 2012. PMID: 22366723
-
Stabilizing the Hsp70-Tau Complex Promotes Turnover in Models of Tauopathy.Cell Chem Biol. 2016 Aug 18;23(8):992-1001. doi: 10.1016/j.chembiol.2016.04.014. Epub 2016 Aug 4. Cell Chem Biol. 2016. PMID: 27499529 Free PMC article.
-
A decade of tau transgenic animal models and beyond.Brain Pathol. 2007 Jan;17(1):91-103. doi: 10.1111/j.1750-3639.2007.00051.x. Brain Pathol. 2007. PMID: 17493043 Free PMC article. Review.
-
Transgenic mouse and cell culture models demonstrate a lack of mechanistic connection between endoplasmic reticulum stress and tau dysfunction.J Neurosci Res. 2010 Jul;88(9):1951-61. doi: 10.1002/jnr.22359. J Neurosci Res. 2010. PMID: 20143409 Free PMC article.
-
Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies?Oxid Med Cell Longev. 2015;2015:151979. doi: 10.1155/2015/151979. Epub 2015 Oct 20. Oxid Med Cell Longev. 2015. PMID: 26576216 Free PMC article. Review.
References
-
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. - PubMed
-
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

