Complex phenotype of mice lacking occludin, a component of tight junction strands
- PMID: 11102513
- PMCID: PMC15062
- DOI: 10.1091/mbc.11.12.4131
Complex phenotype of mice lacking occludin, a component of tight junction strands
Abstract
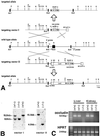
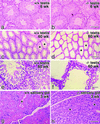
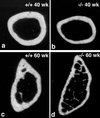
Occludin is an integral membrane protein with four transmembrane domains that is exclusively localized at tight junction (TJ) strands. Here, we describe the generation and analysis of mice carrying a null mutation in the occludin gene. Occludin -/- mice were born with no gross phenotype in the expected Mendelian ratios, but they showed significant postnatal growth retardation. Occludin -/- males produced no litters with wild-type females, whereas occludin -/- females produced litters normally when mated with wild-type males but did not suckle them. In occludin -/- mice, TJs themselves did not appear to be affected morphologically, and the barrier function of intestinal epithelium was normal as far as examined electrophysiologically. However, histological abnormalities were found in several tissues, i.e., chronic inflammation and hyperplasia of the gastric epithelium, calcification in the brain, testicular atrophy, loss of cytoplasmic granules in striated duct cells of the salivary gland, and thinning of the compact bone. These phenotypes suggested that the functions of TJs as well as occludin are more complex than previously supposed.
Figures
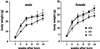



References
-
- Anderson JM, van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. - PubMed
-
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

