Self-association of coilin reveals a common theme in nuclear body localization
- PMID: 11102515
- PMCID: PMC15064
- DOI: 10.1091/mbc.11.12.4159
Self-association of coilin reveals a common theme in nuclear body localization
Abstract
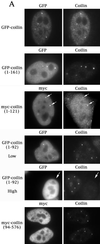
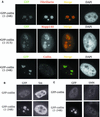
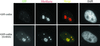
We have found that coilin, the marker protein for Cajal bodies (coiled bodies, CBs), is a self-interacting protein, and we have mapped the domain responsible for this activity to the amino-terminus. Together with a nuclear localization signal, the self-interaction domain is necessary and sufficient for localization to CBs. Overexpression of various wild-type and mutant coilin constructs in HeLa cells results in disruption of both CBs and survival motor neurons (SMN) gems. Additionally, we have identified a cryptic nucleolar localization signal (NoLS), within the coilin protein, which may be exposed in specific coilin phospho-isoforms. The implications of these findings are discussed in light of the fact that other proteins known to localize within nuclear bodies (e. g., PML, SMN and Sam68) can also self-associate. Thus protein self-interaction appears to be a general feature of nuclear body marker proteins.
Figures






References
-
- Cajal SRy. Un sencillo metodo de coloracion selectiva del reticulo protoplasmico y sus efectos en los diversos organos nerviosos de vertebrados y invertebrados. Trab Lab Invest Biol (Madrid) 1903;2:129–221.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

