Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production
- PMID: 11102528
- PMCID: PMC15077
- DOI: 10.1091/mbc.11.12.4347
Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production
Abstract
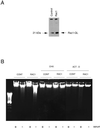
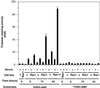
Rho proteins, members of the Ras superfamily of GTPases, are critical elements in signal transduction pathways governing cell proliferation and cell death. Different members of the family of human Rho GTPases, including RhoA, RhoC, and Rac1, participate in the regulation of apoptosis in response to cytokines and serum deprivation in different cell systems. Here, we have characterized the mechanism of apoptosis induced by Rac1 in NIH 3T3 cells. It requires protein synthesis and caspase-3 activity, but it is independent of the release of cytochrome c from mitochondria. Moreover, an increase in mitochondria membrane potential and the production of reactive oxygen species was observed. Rac1-induced apoptosis was related to the simultaneous increase in ceramide production and synthesis of FasL. Generation of FasL may be mediated by transcriptional regulation involving both c-Jun amino terminal kinase as well as nuclear factor-kappa B-dependent signals. None of these signals, ceramides or FasL, was sufficient to induce apoptosis in the parental cell line, NIH 3T3 cells. However, any of them was sufficient to induce apoptosis in the Rac1-expressing cells. Finally, inhibition of FasL signaling drastically reduced apoptosis by Rac1. Thus, Rac1 seems to induce apoptosis by a complex mechanism involving the generation of ceramides and the de novo synthesis of FasL. These results suggest that apoptosis mediated by Rac1 results from a signaling mechanism that involves biochemical and transcriptional events under control of Rac1.
Figures







References
-
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. - PubMed
-
- Basu S, Kolesnick R. Stress signals for apoptosis, ceramide and c-Jun kinase. Oncogene. 1998;17:3277–3285. - PubMed
-
- Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. - PubMed
-
- Boldin MP, Mett IL, Varfolomeev EE, Chumakow Y, Shemer-Avin Y, Camonis JH, Wallach D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995;270:387–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

