Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice
- PMID: 11104792
- PMCID: PMC381466
- DOI: 10.1172/JCI10557
Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice
Abstract
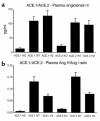
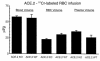
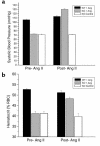
While nephrologists often observe reduced hematocrit associated with inhibitors of angiotensin-converting enzyme (ACE), the basis for this effect is not well understood. We now report that two strains of ACE knockout mice have a normocytic anemia associated with elevated plasma erythropoietin levels. (51)Cr labeling of red cells showed that the knockout mice have a normal total blood volume but a reduced red cell mass. ACE knockout mice, which lack tissue ACE, are anemic despite having normal renal function. These mice have increased plasma levels of the peptide acetyl-SDKP, a possible stem cell suppressor. However, they also show low plasma levels of angiotensin II. Infusion of angiotensin II for 2 weeks increased hematocrit to near normal levels. These data suggest that angiotensin II facilitates erythropoiesis, a conclusion with implications for the management of chronically ill patients on inhibitors of the renin-angiotensin system.
Figures



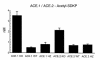
References
-
- Vlahakos DV, et al. Association between activation of the renin-angiotensin system and secondary erythrocytosis in patients with chronic obstructive pulmonary disease. Am J Med. 1999;106:158–164. - PubMed
-
- Jensen JD, Eiskjaer H, Bagger JP, Pedersen EB. Elevated serum level of erythropoietin in congestive heart failure related to renal function. J Intern Med. 1993;233:125–130. - PubMed
-
- Julian BA, et al. Erythropoiesis after withdrawal of enalapril in post-transplant erythrocytosis. Kidney Int. 1994;46:1397–1403. - PubMed
-
- Macdougall IC. The role of ACE inhibitors and angiotensin II receptor blockers in the response to epoetin. Nephrol Dial Transplant. 1999;14:1836–1841. - PubMed
-
- Cruz DN, Perazella MA, Abu-Alfa AK, Mahnensmith RL. Angiotensin-converting enzyme inhibitor therapy in chronic hemodialysis patients: any evidence of erythropoietin resistance? Am J Kidney Dis. 1996;28:535–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

