Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing
- PMID: 11104795
- PMCID: PMC381469
- DOI: 10.1172/JCI11182
Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing
Abstract
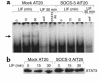
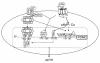
Neuroendocrine ACTH secretion responds to peripheral inflammatory and stress signals. We previously demonstrated that the proinflammatory cytokine, leukemia inhibitory factor (LIF), affects the hypothalamo-pituitary-adrenal axis (HPA) by stimulating in vitro and in vivo pituitary proopiomelanocortin (POMC) gene expression and ACTH secretion and by potentiating the action of hypothalamic corticotropin releasing hormone (CRH). Whereas pathways shown thus far to regulate POMC expression exclusively involve cAMP or calcium, we here describe a direct and indirect STAT3-dependent regulation of POMC transcription by LIF. Using progressive 5'-deletions of POMC promoter, we identified a LIF-responsive -407/-301 region that contains two juxtaposed sequences within -399/-379 related to a STAT3 DNA-binding motif. Each sequence within -399/-379 separately corresponds to a low-affinity and direct binding site for STAT3, but, in combination, these sequences bind STAT3 cooperatively and with high affinity. Moreover, LIF-activated STAT3 indirectly mediates LIF corticotroph action by inducing and potentiating CRH-induced c-fos and JunB expression and binding to the POMC AP-1 element. We therefore conclude that both a direct and indirect route mediate LIF-induced STAT3 activation of POMC transcription. Demonstration of STAT3-dependent regulation of the POMC gene represents a powerful mechanism for immuno-neuroendocrine interfacing and implies a direct stimulation of ACTH secretion by inflammatory and stress-derived STAT3-inducing cytokines.
Figures

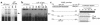

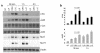


References
-
- Auernhammer CJ, Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev. 2000;21:313–345. - PubMed
-
- Wang Z, Ren SG, Melmed S. Hypothalamic and pituitary LIF gene expression in-vivo: a novel endotoxin-inducible neuroendocrine interface. Endocrinology. 1996;137:2947–2953. - PubMed
-
- Chesnokova V, Auernhammer CJ, Melmed S. Murine LIF gene disruption attenuates the hypothalamo-pituitary-adrenal axis stress response. Endocrinology. 1998;139:2209–2216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

