Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring
- PMID: 11106350
- PMCID: PMC7110382
- DOI: 10.1093/clinchem/46.12.2050
Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring
Abstract
Purpose: To review information on the use of laboratory tests in screening, diagnosis, and monitoring of acute and chronic hepatic injury.
Data sources and study selection: A MEDLINE search was performed for key words related to hepatic diseases, including acute hepatitis, chronic hepatitis, alcoholic hepatitis, cirrhosis, hepatocellular carcinoma, and etiologic causes. Abstracts were reviewed, and articles discussing use of laboratory tests selected for review. Additional articles were selected from the references. Guideline Preparation and Review: Drafts of the guidelines were posted on the Internet, presented at the AACC Annual Meeting in 1999, and reviewed by experts. Areas requiring further amplification or literature review were identified for further analysis. Specific recommendations were made based on analysis of published data and evaluated for strength of evidence and clinical impact.
Recommendations: Although many specific recommendations are made in the guidelines, only some summary recommendations are listed here. In acute hepatic injury, prothrombin time and, to a lesser extent, total bilirubin are the best indicators of severity of disease. Although ALT is useful for detecting acute and chronic hepatic injury, it is not related to severity of acute hepatic injury and only weakly related to severity of chronic hepatic injury. Specific tests of viral markers should be the initial differential tests in both acute and chronic hepatic injury; when positive, they are also useful for monitoring recovery from hepatitis B and C.
Figures
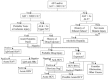
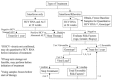
Comment in
-
Is it necessary to order aspartate aminotransferase with alanine aminotransferase in clinical practice?Clin Chem. 2001 Jun;47(6):1133-5. Clin Chem. 2001. PMID: 11375313 No abstract available.
References
-
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 2000;46:2027-2049. - PubMed
-
- Ellis G, Goldberg DM, Spooner RJ, Ward AM. Serum enzyme tests in diseases of the liver and biliary tree. Am J Clin Pathol 1978;70:248-258. - PubMed
-
- Rozen P, Korn RJ, Zimmerman HJ. Computer analysis of liver function tests and their interrelationship in 347 cases of viral hepatitis. Isr J Med Sci 1970;6:67-79. - PubMed
-
- Anciaux ML, Pelletier AG, Attali P, Meduri B, Liguory C, Etienne JP. Prospective study of clinical and biochemical features of symptomatic choledocholithiasis. Dig Dis Sci 1986;31:449-453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

