Maternal germ-line transmission of mutant mtDNAs from embryonic stem cell-derived chimeric mice
- PMID: 11106380
- PMCID: PMC18941
- DOI: 10.1073/pnas.250491597
Maternal germ-line transmission of mutant mtDNAs from embryonic stem cell-derived chimeric mice
Abstract
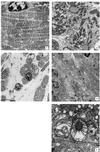
We report a method for introducing mtDNA mutations into the mouse female germ line by means of embryonic stem (ES) cell cybrids. Mitochondria were recovered from the brain of a NZB mouse by fusion of synaptosomes to a mtDNA-deficient (rho degrees ) cell line. These cybrids were enucleated and the cytoplasts were electrofused to rhodamine-6G (R-6G)-treated female ES cells. The resulting ES cell cybrids permitted transmission of the NZB mtDNAs through the mouse maternal lineage for three generations. Similarly, mtDNAs from a partially respiratory-deficient chloramphenicol-resistant (CAP(R)) cell line also were introduced into female chimeric mice and were transmitted to the progeny. CAP(R) chimeric mice developed a variety of ocular abnormalities, including congenital cataracts, decreased retinal function, and hamaratomas of the optic nerve. The germ-line transmission of the CAP(R) mutation resulted in animals with growth retardation, myopathy, dilated cardiomyopathy, and perinatal or in utero lethality. Skeletal and heart muscle mitochondria of the CAP(R) mice were enlarged and atypical with inclusions. This mouse ES cell-cybrid approach now provides the means to generate a wide variety of mouse models of mitochondrial disease.
Figures

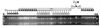

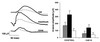




Comment in
-
Transmitochondrial mice: proof of principle and promises.Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):401-3. doi: 10.1073/pnas.98.2.401. Proc Natl Acad Sci U S A. 2001. PMID: 11209045 Free PMC article. No abstract available.
References
-
- Li Y, Huang T T, Carlson E J, Melov S, Ursell P C, Olson J L, Noble L J, Yoshimura M P, Berger C, Chan P H, et al. Nat Genet. 1995;11:376–381. - PubMed
-
- Graham B, Waymire K, Cottrell B, Trounce I A, MacGregor G R, Wallace D C. Nat Genet. 1997;16:226–234. - PubMed
-
- Melov S, Schneider J A, Day B J, Hinerfeld D, Coskun P, Mirra S S, Crapo J D, Wallace D C. Nat Genet. 1998;18:159–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

