Species-specific polyamines from diatoms control silica morphology
- PMID: 11106386
- PMCID: PMC18883
- DOI: 10.1073/pnas.260496497
Species-specific polyamines from diatoms control silica morphology
Abstract
Biomineralizing organisms use organic molecules to generate species-specific mineral patterns. Here, we describe the chemical structure of long-chain polyamines (up to 20 repeated units), which represent the main organic constituent of diatom biosilica. These substances are the longest polyamine chains found in nature and induce rapid silica precipitation from a silicic acid solution. Each diatom is equipped with a species-specific set of polyamines and silica-precipitating proteins, which are termed silaffins. Different morphologies of precipitating silica can be generated by polyamines of different chain lengths as well as by a synergistic action of long-chain polyamines and silaffins.
Figures

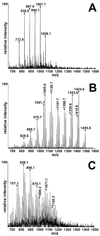




References
-
- Lowenstam H A. Science. 1981;211:1126–1131. - PubMed
-
- Volcani B E. In: Silicon and Siliceous Structures in Biological Systems. Simpson T L, Volcani B E, editors. New York: Springer; 1981. pp. 157–200.
-
- Pickett-Heaps J, Schmid A M M, Edgar L A. In: Progress in Phycological Research. Round F E, Chapman D J, editors. Vol. 7. Bristol, U.K.: Biopress; 1990. pp. 1–169.
-
- Gordon R, Drum R W. Int Rev Cytol. 1994;150:243–372. - PubMed
-
- Mann S. Nature (London) 1993;365:499–505.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

