Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses
- PMID: 11106387
- PMCID: PMC18963
- DOI: 10.1073/pnas.260497597
Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses
Abstract
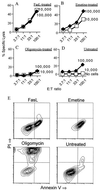
General immunostimulants (adjuvants) are essential for generating immunity to many antigens. In bacterial infections, adjuvants are provided by components of the microorganism, e.g., lipopolysaccharide. However, it is unclear what provides the adjuvant effect for immune responses that are generated to tumors and many viruses. Here we show that cell injury and death of tumor or even normal cells provide a potent adjuvant effect for the stimulation of cytotoxic T lymphocyte responses. This adjuvant activity is constitutively present in the cytoplasm of cells and is increased in the cytoplasm of cells dying by apoptosis. The release of these components stimulates immune responses both locally and at a distance, and provides a simple mechanism to alert the immune system to potential danger in almost all pathological situations.
Figures


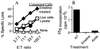


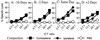
References
-
- Janeway C A., Jr Cold Spring Harbor Symp Quant Biol. 1989;54:1–13. - PubMed
-
- Medzhitov R, Janeway C., Jr Immunol Rev. 2000;173:89–97. - PubMed
-
- Janeway C A., Jr Immunol Today. 1992;13:11–16. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. Cell. 1996;86:973–983. - PubMed
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Immunity. 1998;9:143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

